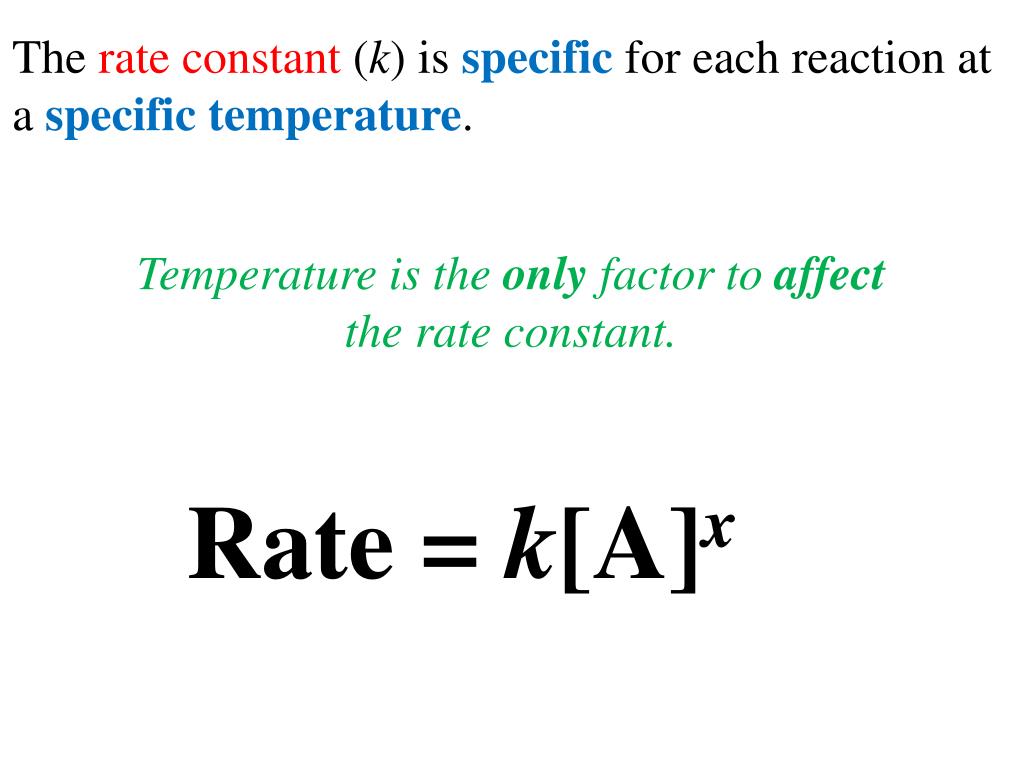
Rate Constant Reaction K . Rate = k [a] the units for the rate are mol/l. The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. The general rate law is usually expressed as: The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. = \;\frac { { {\rm. \[ \text{rate} = k[a]^s[b]^t \label{2} \] The rate constant is equal to: A rate constant, \(k\), is a proportionality constant for a given reaction. The specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations of reactants.
from www.slideserve.com
The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. The rate constant is equal to: \[ \text{rate} = k[a]^s[b]^t \label{2} \] = \;\frac { { {\rm. A rate constant, \(k\), is a proportionality constant for a given reaction. The general rate law is usually expressed as: The specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations of reactants. The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. Rate = k [a] the units for the rate are mol/l.
PPT Rate Laws PowerPoint Presentation, free download ID4905523
Rate Constant Reaction K Rate = k [a] the units for the rate are mol/l. The general rate law is usually expressed as: The rate constant is equal to: Rate = k [a] the units for the rate are mol/l. The specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations of reactants. The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. = \;\frac { { {\rm. A rate constant, \(k\), is a proportionality constant for a given reaction. The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. \[ \text{rate} = k[a]^s[b]^t \label{2} \]
From byjus.com
The rate constant of a reaction is 1.2×10^ 3sec^ 1 at 30℃and 2.1×10 Rate Constant Reaction K = \;\frac { { {\rm. The specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations of reactants. The general rate law is usually expressed as: The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as.. Rate Constant Reaction K.
From www.slideserve.com
PPT CHEMICAL PowerPoint Presentation ID5609537 Rate Constant Reaction K \[ \text{rate} = k[a]^s[b]^t \label{2} \] The rate constant is equal to: The specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations of reactants. The general rate law is usually expressed as: = \;\frac { { {\rm. Rate = k [a] the units for the rate are mol/l. The rate. Rate Constant Reaction K.
From www.slideserve.com
PPT Rate laws (2) PowerPoint Presentation, free download ID2351800 Rate Constant Reaction K The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. A rate constant, \(k\), is a proportionality constant for a given reaction. The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction. Rate Constant Reaction K.
From www.chegg.com
Solved The temperature dependence of the reaction rate Rate Constant Reaction K The general rate law is usually expressed as: The specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations of reactants. \[ \text{rate} = k[a]^s[b]^t \label{2} \] The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes. Rate Constant Reaction K.
From www.slideshare.net
Chapter 14 Lecture Chemical Rate Constant Reaction K = \;\frac { { {\rm. The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. Rate = k [a] the units for the rate are mol/l. A rate constant, \(k\), is a proportionality constant for a given reaction. The specific rate constant \(\left( k \right)\). Rate Constant Reaction K.
From www.slideserve.com
PPT ERT 316 REACTION ENGINEERING CHAPTER 3 RATE LAWS & STOICHIOMETRY Rate Constant Reaction K The rate constant is equal to: = \;\frac { { {\rm. The general rate law is usually expressed as: The specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations of reactants. The rate constant k and the reaction orders m and n must be determined experimentally by observing how the. Rate Constant Reaction K.
From www.chemistrystudent.com
The Rate Equation (ALevel) ChemistryStudent Rate Constant Reaction K The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. = \;\frac { { {\rm. The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. The general rate law is. Rate Constant Reaction K.
From www.numerade.com
SOLVED The rate constant k for a certain reaction is measured at two Rate Constant Reaction K The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. The general rate law is usually expressed as: A rate. Rate Constant Reaction K.
From byjus.com
What is the unit of rate constant for first order reaction Rate Constant Reaction K = \;\frac { { {\rm. \[ \text{rate} = k[a]^s[b]^t \label{2} \] The general rate law is usually expressed as: A rate constant, \(k\), is a proportionality constant for a given reaction. The rate constant is equal to: The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction. Rate Constant Reaction K.
From www.numerade.com
SOLVEDConsider the following reaction, where the rate constant k Rate Constant Reaction K The general rate law is usually expressed as: = \;\frac { { {\rm. The rate constant is equal to: The specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations of reactants. \[ \text{rate} = k[a]^s[b]^t \label{2} \] Rate = k [a] the units for the rate are mol/l. A rate. Rate Constant Reaction K.
From chem.libretexts.org
Chapter 13.4 Using Graphs to Determine Rate Laws, Rate Constants and Rate Constant Reaction K A rate constant, \(k\), is a proportionality constant for a given reaction. The general rate law is usually expressed as: The specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations of reactants. = \;\frac { { {\rm. The rate constant k and the reaction orders m and n must be. Rate Constant Reaction K.
From newbedev.com
Chemistry Formula for rate constant for the first order reaction Rate Constant Reaction K A rate constant, \(k\), is a proportionality constant for a given reaction. The specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations of reactants. The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. Rate. Rate Constant Reaction K.
From www.toppr.com
Derive an integrated rate equation for rate constant of a zero order Rate Constant Reaction K Rate = k [a] the units for the rate are mol/l. The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. = \;\frac { { {\rm. The general rate law is usually expressed as: The rate constant k and the reaction orders m and n. Rate Constant Reaction K.
From chemistryguru.com.sg
Rate Equation and Order of Reaction Rate Constant Reaction K The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. The rate constant is equal to: Rate = k [a] the units for the rate are mol/l. A rate constant, \(k\), is a proportionality constant for a given reaction. The specific rate constant \(\left( k. Rate Constant Reaction K.
From www.pinterest.com
Specific Rate Constant definition A constant relating the Rate Constant Reaction K The specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations of reactants. The rate constant is equal to: = \;\frac { { {\rm. The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. The general. Rate Constant Reaction K.
From www.slideserve.com
PPT Rate Laws PowerPoint Presentation, free download ID4905523 Rate Constant Reaction K \[ \text{rate} = k[a]^s[b]^t \label{2} \] = \;\frac { { {\rm. The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. The specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations of reactants. Rate =. Rate Constant Reaction K.
From www.youtube.com
Integrated Rate Laws Zero, First, & Second Order Reactions Chemical Rate Constant Reaction K The rate constant is equal to: The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. A rate constant, \(k\),. Rate Constant Reaction K.
From www.youtube.com
Determine the rate constant (k) for a reaction YouTube Rate Constant Reaction K \[ \text{rate} = k[a]^s[b]^t \label{2} \] The rate constant is equal to: Rate = k [a] the units for the rate are mol/l. The general rate law is usually expressed as: The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. = \;\frac { {. Rate Constant Reaction K.
From www.numerade.com
SOLVED Which one of the following given graphs represents the Rate Constant Reaction K Rate = k [a] the units for the rate are mol/l. A rate constant, \(k\), is a proportionality constant for a given reaction. \[ \text{rate} = k[a]^s[b]^t \label{2} \] The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. The rate constant k and the. Rate Constant Reaction K.
From general.chemistrysteps.com
FirstOrder Reactions Chemistry Steps Rate Constant Reaction K \[ \text{rate} = k[a]^s[b]^t \label{2} \] = \;\frac { { {\rm. The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. A rate constant, \(k\), is a proportionality constant for a given reaction. The general rate law is usually expressed as: Rate = k [a]. Rate Constant Reaction K.
From www.youtube.com
The rate constant for a first order reaction six times when the Rate Constant Reaction K The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. Rate = k [a] the units for the rate are mol/l. The general rate law is usually expressed as: The rate constant k and the reaction orders m and n must be determined experimentally by. Rate Constant Reaction K.
From facts.net
14 Fascinating Facts About Rate Constant Rate Constant Reaction K The general rate law is usually expressed as: \[ \text{rate} = k[a]^s[b]^t \label{2} \] Rate = k [a] the units for the rate are mol/l. The rate constant is equal to: The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. The rate constant k. Rate Constant Reaction K.
From www.youtube.com
Integrated Rate Equation for third Order Reaction Third Order Rate Constant Reaction K The rate constant is equal to: = \;\frac { { {\rm. \[ \text{rate} = k[a]^s[b]^t \label{2} \] A rate constant, \(k\), is a proportionality constant for a given reaction. The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. The rate constant k and the. Rate Constant Reaction K.
From askfilo.com
Rate constant k for a first order reaction has been found to be 2.54×10−3.. Rate Constant Reaction K = \;\frac { { {\rm. The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. Rate = k [a] the units for the rate are mol/l. The rate constant k and the reaction orders m and n must be determined experimentally by observing how the. Rate Constant Reaction K.
From www.chegg.com
Solved For a secondorder reaction, the rate constant k is Rate Constant Reaction K The specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations of reactants. The general rate law is usually expressed as: A rate constant, \(k\), is a proportionality constant for a given reaction. \[ \text{rate} = k[a]^s[b]^t \label{2} \] The rate constant k and the reaction orders m and n must. Rate Constant Reaction K.
From www.youtube.com
Intro to Rate Laws, Rate Constants, Reaction Order Chemistry Tutorial Rate Constant Reaction K = \;\frac { { {\rm. The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. A rate constant, \(k\), is. Rate Constant Reaction K.
From www.youtube.com
Writing Rate Laws of Reaction Mechanisms Using The Rate Determining Rate Constant Reaction K The rate constant is equal to: The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. Rate = k [a] the units for the rate are mol/l. The rate constant k and the reaction orders m and n must be determined experimentally by observing how. Rate Constant Reaction K.
From www.toppr.com
The unit of rate constant for a zero order reaction is Rate Constant Reaction K The rate constant is equal to: The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. A rate constant, \(k\), is a proportionality constant for a given reaction. \[ \text{rate} = k[a]^s[b]^t \label{2} \] Rate = k [a] the units for the rate are mol/l.. Rate Constant Reaction K.
From www.slideserve.com
PPT SCH4U Unit 1 Energy Changes & Rates of Reactions (Cont’d Rate Constant Reaction K The general rate law is usually expressed as: The rate constant is equal to: = \;\frac { { {\rm. The specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations of reactants. Rate = k [a] the units for the rate are mol/l. The rate constant k and the reaction orders. Rate Constant Reaction K.
From www.toppr.com
Units of rate constant of a first order reaction is Rate Constant Reaction K The general rate law is usually expressed as: A rate constant, \(k\), is a proportionality constant for a given reaction. = \;\frac { { {\rm. The rate constant is equal to: \[ \text{rate} = k[a]^s[b]^t \label{2} \] The specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations of reactants. Rate. Rate Constant Reaction K.
From www.youtube.com
How To Determine The Units Of The Rate Constant K Chemical Rate Constant Reaction K The general rate law is usually expressed as: Rate = k [a] the units for the rate are mol/l. The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. The rate constant is equal to: The specific rate constant \(\left( k \right)\) is the proportionality. Rate Constant Reaction K.
From www.chegg.com
Solved The rate constant k for a certain reaction is Rate Constant Reaction K The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. The general rate law is usually expressed as: The specific rate constant \(\left( k \right)\) is the proportionality constant relating the rate of the reaction to the concentrations of reactants. The rate constant k and. Rate Constant Reaction K.
From www.sliderbase.com
Determining Order with Concentration vs. Time data Rate Constant Reaction K The rate constant is equal to: The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. Rate = k [a]. Rate Constant Reaction K.
From www.tessshebaylo.com
Rate Constant Equation For Zero Order Tessshebaylo Rate Constant Reaction K The rate constant k and the reaction orders m and n must be determined experimentally by observing how the rate of a reaction changes as. Rate = k [a] the units for the rate are mol/l. A rate constant, \(k\), is a proportionality constant for a given reaction. The rate constant is equal to: = \;\frac { { {\rm. The. Rate Constant Reaction K.
From www.sliderbase.com
Determining Order with Concentration vs. Time data Rate Constant Reaction K Rate = k [a] the units for the rate are mol/l. The general rate law is usually expressed as: A rate constant, \(k\), is a proportionality constant for a given reaction. \[ \text{rate} = k[a]^s[b]^t \label{2} \] The rate constant is equal to: = \;\frac { { {\rm. The specific rate constant \(\left( k \right)\) is the proportionality constant relating. Rate Constant Reaction K.