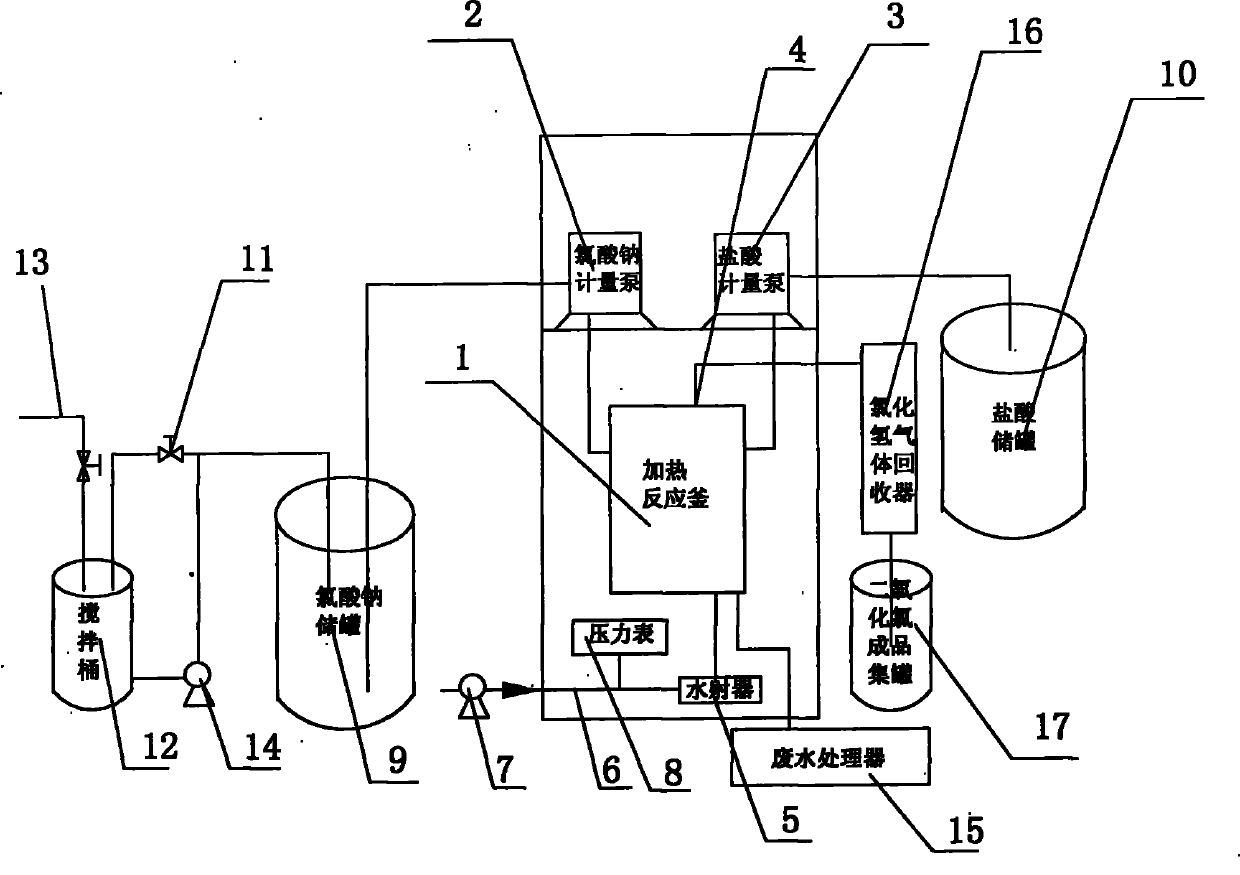
Chlorine Dioxide Production . Production methods involve either the reduction of chlorate or the oxidation of chlorite. Chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. Results demonstrate that suitable reagents can be produced by electrolyzing 20 g l −1 sodium chloride solutions at 50 ma cm −2 and 50 °c, and 3000 mg l −1 naclo 4 solutions at 5.0 ma cm −2 and 15 °c with current efficiencies of 30.9% and 48.0%, respectively. Both of these starting materials are relatively stable, despite their oxidizing potential. Chlorine dioxide (clo 2) is produced from either sodium chlorite (naclo 2) or sodium chlorate (naclo 3). Clo 2 treatment can prevent methionine conversion, and eliminate ethylene and other substances quickly and effectively (fu and.
from eureka.patsnap.com
Chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. Both of these starting materials are relatively stable, despite their oxidizing potential. Results demonstrate that suitable reagents can be produced by electrolyzing 20 g l −1 sodium chloride solutions at 50 ma cm −2 and 50 °c, and 3000 mg l −1 naclo 4 solutions at 5.0 ma cm −2 and 15 °c with current efficiencies of 30.9% and 48.0%, respectively. Clo 2 treatment can prevent methionine conversion, and eliminate ethylene and other substances quickly and effectively (fu and. Chlorine dioxide (clo 2) is produced from either sodium chlorite (naclo 2) or sodium chlorate (naclo 3). Production methods involve either the reduction of chlorate or the oxidation of chlorite.
Chlorine dioxide generating system Eureka Patsnap develop
Chlorine Dioxide Production Results demonstrate that suitable reagents can be produced by electrolyzing 20 g l −1 sodium chloride solutions at 50 ma cm −2 and 50 °c, and 3000 mg l −1 naclo 4 solutions at 5.0 ma cm −2 and 15 °c with current efficiencies of 30.9% and 48.0%, respectively. Production methods involve either the reduction of chlorate or the oxidation of chlorite. Chlorine dioxide (clo 2) is produced from either sodium chlorite (naclo 2) or sodium chlorate (naclo 3). Clo 2 treatment can prevent methionine conversion, and eliminate ethylene and other substances quickly and effectively (fu and. Both of these starting materials are relatively stable, despite their oxidizing potential. Results demonstrate that suitable reagents can be produced by electrolyzing 20 g l −1 sodium chloride solutions at 50 ma cm −2 and 50 °c, and 3000 mg l −1 naclo 4 solutions at 5.0 ma cm −2 and 15 °c with current efficiencies of 30.9% and 48.0%, respectively. Chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media.
From www.dreamstime.com
MMS CHLORINE DIOXIDE PRODUCTION Stock Image Image of drip, diet Chlorine Dioxide Production Both of these starting materials are relatively stable, despite their oxidizing potential. Chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. Clo 2 treatment can prevent methionine conversion, and eliminate ethylene and other substances quickly and effectively (fu and. Production methods involve either the reduction of chlorate or the oxidation of chlorite. Results demonstrate that. Chlorine Dioxide Production.
From healingoracle.ch
THE HISTORY OF CHLORINE DIOXIDE Healing Oracle Chlorine Dioxide Production Chlorine dioxide (clo 2) is produced from either sodium chlorite (naclo 2) or sodium chlorate (naclo 3). Results demonstrate that suitable reagents can be produced by electrolyzing 20 g l −1 sodium chloride solutions at 50 ma cm −2 and 50 °c, and 3000 mg l −1 naclo 4 solutions at 5.0 ma cm −2 and 15 °c with current. Chlorine Dioxide Production.
From www.dioxide.com
Electrochemical Chlorine Generator Electrochlorination from brine (salt) Chlorine Dioxide Production Results demonstrate that suitable reagents can be produced by electrolyzing 20 g l −1 sodium chloride solutions at 50 ma cm −2 and 50 °c, and 3000 mg l −1 naclo 4 solutions at 5.0 ma cm −2 and 15 °c with current efficiencies of 30.9% and 48.0%, respectively. Clo 2 treatment can prevent methionine conversion, and eliminate ethylene and. Chlorine Dioxide Production.
From eathisa.com
Chlorine Dioxide Generator Chlorine Dioxide Production Clo 2 treatment can prevent methionine conversion, and eliminate ethylene and other substances quickly and effectively (fu and. Results demonstrate that suitable reagents can be produced by electrolyzing 20 g l −1 sodium chloride solutions at 50 ma cm −2 and 50 °c, and 3000 mg l −1 naclo 4 solutions at 5.0 ma cm −2 and 15 °c with. Chlorine Dioxide Production.
From www.google.com
Patent EP0284577B1 Process for production of chlorine dioxide Chlorine Dioxide Production Both of these starting materials are relatively stable, despite their oxidizing potential. Production methods involve either the reduction of chlorate or the oxidation of chlorite. Chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. Chlorine dioxide (clo 2) is produced from either sodium chlorite (naclo 2) or sodium chlorate (naclo 3). Clo 2 treatment can. Chlorine Dioxide Production.
From www.mdpi.com
Catalysts Free FullText Full and Sustainable Electrochemical Chlorine Dioxide Production Both of these starting materials are relatively stable, despite their oxidizing potential. Results demonstrate that suitable reagents can be produced by electrolyzing 20 g l −1 sodium chloride solutions at 50 ma cm −2 and 50 °c, and 3000 mg l −1 naclo 4 solutions at 5.0 ma cm −2 and 15 °c with current efficiencies of 30.9% and 48.0%,. Chlorine Dioxide Production.
From www.pall.com
Chlorine Dioxide Pulp Bleaching & Paper Chemical Preparation Pall Chlorine Dioxide Production Production methods involve either the reduction of chlorate or the oxidation of chlorite. Chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. Chlorine dioxide (clo 2) is produced from either sodium chlorite (naclo 2) or sodium chlorate (naclo 3). Results demonstrate that suitable reagents can be produced by electrolyzing 20 g l −1 sodium chloride. Chlorine Dioxide Production.
From www.researchgate.net
(PDF) Production and Analysis of Chlorine Dioxide Chlorine Dioxide Production Clo 2 treatment can prevent methionine conversion, and eliminate ethylene and other substances quickly and effectively (fu and. Results demonstrate that suitable reagents can be produced by electrolyzing 20 g l −1 sodium chloride solutions at 50 ma cm −2 and 50 °c, and 3000 mg l −1 naclo 4 solutions at 5.0 ma cm −2 and 15 °c with. Chlorine Dioxide Production.
From www.dioxide.com
Chlorine Dioxide Generation and Dosing Systems chemical and echem Chlorine Dioxide Production Production methods involve either the reduction of chlorate or the oxidation of chlorite. Clo 2 treatment can prevent methionine conversion, and eliminate ethylene and other substances quickly and effectively (fu and. Results demonstrate that suitable reagents can be produced by electrolyzing 20 g l −1 sodium chloride solutions at 50 ma cm −2 and 50 °c, and 3000 mg l. Chlorine Dioxide Production.
From www.seawaterchlorination.com
Industrial Chlorine Cdioxide Generators Supplier, Chlorine Dioxide Gas Chlorine Dioxide Production Results demonstrate that suitable reagents can be produced by electrolyzing 20 g l −1 sodium chloride solutions at 50 ma cm −2 and 50 °c, and 3000 mg l −1 naclo 4 solutions at 5.0 ma cm −2 and 15 °c with current efficiencies of 30.9% and 48.0%, respectively. Chlorine dioxide (clo 2) is produced from either sodium chlorite (naclo. Chlorine Dioxide Production.
From www.dreamstime.com
MMS CHLORINE DIOXIDE PRODUCTION with DOSING TOOLS Stock Image Image Chlorine Dioxide Production Chlorine dioxide (clo 2) is produced from either sodium chlorite (naclo 2) or sodium chlorate (naclo 3). Both of these starting materials are relatively stable, despite their oxidizing potential. Results demonstrate that suitable reagents can be produced by electrolyzing 20 g l −1 sodium chloride solutions at 50 ma cm −2 and 50 °c, and 3000 mg l −1 naclo. Chlorine Dioxide Production.
From www.google.com
Patent US20120294794 Process for the Production of Chlorine Dioxide Chlorine Dioxide Production Both of these starting materials are relatively stable, despite their oxidizing potential. Chlorine dioxide (clo 2) is produced from either sodium chlorite (naclo 2) or sodium chlorate (naclo 3). Clo 2 treatment can prevent methionine conversion, and eliminate ethylene and other substances quickly and effectively (fu and. Production methods involve either the reduction of chlorate or the oxidation of chlorite.. Chlorine Dioxide Production.
From www.dioxide.com
3Chemical Chlorine Dioxide Generators High Conversion Chlorine Dioxide Production Production methods involve either the reduction of chlorate or the oxidation of chlorite. Chlorine dioxide (clo 2) is produced from either sodium chlorite (naclo 2) or sodium chlorate (naclo 3). Results demonstrate that suitable reagents can be produced by electrolyzing 20 g l −1 sodium chloride solutions at 50 ma cm −2 and 50 °c, and 3000 mg l −1. Chlorine Dioxide Production.
From www.dioxide.com
Electricide Electrochemical Chlorine Dioxide Generator Chlorine Dioxide Production Both of these starting materials are relatively stable, despite their oxidizing potential. Production methods involve either the reduction of chlorate or the oxidation of chlorite. Chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. Results demonstrate that suitable reagents can be produced by electrolyzing 20 g l −1 sodium chloride solutions at 50 ma cm. Chlorine Dioxide Production.
From www.dreamstime.com
Chlorine Dioxide, ClO2, Ballandstick Model, Molecular and Chemical Chlorine Dioxide Production Chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. Chlorine dioxide (clo 2) is produced from either sodium chlorite (naclo 2) or sodium chlorate (naclo 3). Production methods involve either the reduction of chlorate or the oxidation of chlorite. Clo 2 treatment can prevent methionine conversion, and eliminate ethylene and other substances quickly and effectively. Chlorine Dioxide Production.
From www.google.com
Patent US7452511 Reactor for production of chlorine dioxide, methods Chlorine Dioxide Production Clo 2 treatment can prevent methionine conversion, and eliminate ethylene and other substances quickly and effectively (fu and. Production methods involve either the reduction of chlorate or the oxidation of chlorite. Results demonstrate that suitable reagents can be produced by electrolyzing 20 g l −1 sodium chloride solutions at 50 ma cm −2 and 50 °c, and 3000 mg l. Chlorine Dioxide Production.
From www.nouryon.com
Eka ClO2 chlorine dioxide technologies Chlorine Dioxide Production Clo 2 treatment can prevent methionine conversion, and eliminate ethylene and other substances quickly and effectively (fu and. Production methods involve either the reduction of chlorate or the oxidation of chlorite. Results demonstrate that suitable reagents can be produced by electrolyzing 20 g l −1 sodium chloride solutions at 50 ma cm −2 and 50 °c, and 3000 mg l. Chlorine Dioxide Production.
From feedwater.co.uk
Chlorine Dioxide Water Treatment Feedwater site Chlorine Dioxide Production Clo 2 treatment can prevent methionine conversion, and eliminate ethylene and other substances quickly and effectively (fu and. Chlorine dioxide (clo 2) is produced from either sodium chlorite (naclo 2) or sodium chlorate (naclo 3). Production methods involve either the reduction of chlorate or the oxidation of chlorite. Chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in. Chlorine Dioxide Production.
From idiclo2.com
About Chlorine Dioxide International Dioxide Chlorine Dioxide Production Chlorine dioxide (clo 2) is produced from either sodium chlorite (naclo 2) or sodium chlorate (naclo 3). Clo 2 treatment can prevent methionine conversion, and eliminate ethylene and other substances quickly and effectively (fu and. Chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. Results demonstrate that suitable reagents can be produced by electrolyzing 20. Chlorine Dioxide Production.
From www.google.com
Patent EP0357198B1 Production of chlorine dioxide Google Patents Chlorine Dioxide Production Chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. Production methods involve either the reduction of chlorate or the oxidation of chlorite. Results demonstrate that suitable reagents can be produced by electrolyzing 20 g l −1 sodium chloride solutions at 50 ma cm −2 and 50 °c, and 3000 mg l −1 naclo 4 solutions. Chlorine Dioxide Production.
From www.frontiersin.org
Frontiers Fresh Produce Safety and Quality Chlorine Dioxide’s Role Chlorine Dioxide Production Both of these starting materials are relatively stable, despite their oxidizing potential. Production methods involve either the reduction of chlorate or the oxidation of chlorite. Results demonstrate that suitable reagents can be produced by electrolyzing 20 g l −1 sodium chloride solutions at 50 ma cm −2 and 50 °c, and 3000 mg l −1 naclo 4 solutions at 5.0. Chlorine Dioxide Production.
From www.youtube.com
Poultry and Pig Chlorine Dioxide Water Treatment YouTube Chlorine Dioxide Production Production methods involve either the reduction of chlorate or the oxidation of chlorite. Both of these starting materials are relatively stable, despite their oxidizing potential. Chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. Chlorine dioxide (clo 2) is produced from either sodium chlorite (naclo 2) or sodium chlorate (naclo 3). Results demonstrate that suitable. Chlorine Dioxide Production.
From www.google.com
Patent US5376350 Plug flow process for the production of chlorine Chlorine Dioxide Production Chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. Production methods involve either the reduction of chlorate or the oxidation of chlorite. Both of these starting materials are relatively stable, despite their oxidizing potential. Clo 2 treatment can prevent methionine conversion, and eliminate ethylene and other substances quickly and effectively (fu and. Results demonstrate that. Chlorine Dioxide Production.
From onenessdrops.com
Understanding Chlorine Dioxide The Science Behind Water Purification Chlorine Dioxide Production Production methods involve either the reduction of chlorate or the oxidation of chlorite. Clo 2 treatment can prevent methionine conversion, and eliminate ethylene and other substances quickly and effectively (fu and. Results demonstrate that suitable reagents can be produced by electrolyzing 20 g l −1 sodium chloride solutions at 50 ma cm −2 and 50 °c, and 3000 mg l. Chlorine Dioxide Production.
From eureka.patsnap.com
Chlorine dioxide generating system Eureka Patsnap develop Chlorine Dioxide Production Chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. Both of these starting materials are relatively stable, despite their oxidizing potential. Production methods involve either the reduction of chlorate or the oxidation of chlorite. Chlorine dioxide (clo 2) is produced from either sodium chlorite (naclo 2) or sodium chlorate (naclo 3). Results demonstrate that suitable. Chlorine Dioxide Production.
From osornosolution-store.ca
Chlorine Dioxide Generation Kit, 1L bottle Osorno Enterprises Chlorine Dioxide Production Results demonstrate that suitable reagents can be produced by electrolyzing 20 g l −1 sodium chloride solutions at 50 ma cm −2 and 50 °c, and 3000 mg l −1 naclo 4 solutions at 5.0 ma cm −2 and 15 °c with current efficiencies of 30.9% and 48.0%, respectively. Clo 2 treatment can prevent methionine conversion, and eliminate ethylene and. Chlorine Dioxide Production.
From www.health.news
Here’s what you need to know about chlorine dioxide (ClO2), the Chlorine Dioxide Production Clo 2 treatment can prevent methionine conversion, and eliminate ethylene and other substances quickly and effectively (fu and. Both of these starting materials are relatively stable, despite their oxidizing potential. Results demonstrate that suitable reagents can be produced by electrolyzing 20 g l −1 sodium chloride solutions at 50 ma cm −2 and 50 °c, and 3000 mg l −1. Chlorine Dioxide Production.
From www.prominent.com
Chlorine Dioxide System Bello Zon CDKd ProMinent Chlorine Dioxide Production Chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. Both of these starting materials are relatively stable, despite their oxidizing potential. Production methods involve either the reduction of chlorate or the oxidation of chlorite. Chlorine dioxide (clo 2) is produced from either sodium chlorite (naclo 2) or sodium chlorate (naclo 3). Clo 2 treatment can. Chlorine Dioxide Production.
From www.wcs-group.co.uk
Chlorine Dioxide Generator Fact Sheet WCS Group Chlorine Dioxide Production Both of these starting materials are relatively stable, despite their oxidizing potential. Chlorine dioxide (clo 2) is produced from either sodium chlorite (naclo 2) or sodium chlorate (naclo 3). Production methods involve either the reduction of chlorate or the oxidation of chlorite. Clo 2 treatment can prevent methionine conversion, and eliminate ethylene and other substances quickly and effectively (fu and.. Chlorine Dioxide Production.
From www.google.de
Patent US8431104 Process for the production of chlorine dioxide Chlorine Dioxide Production Both of these starting materials are relatively stable, despite their oxidizing potential. Clo 2 treatment can prevent methionine conversion, and eliminate ethylene and other substances quickly and effectively (fu and. Chlorine dioxide (clo 2) is produced from either sodium chlorite (naclo 2) or sodium chlorate (naclo 3). Chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic. Chlorine Dioxide Production.
From eureka.patsnap.com
Chlorine dioxide generator for the efficient generation of chlorine Chlorine Dioxide Production Production methods involve either the reduction of chlorate or the oxidation of chlorite. Both of these starting materials are relatively stable, despite their oxidizing potential. Results demonstrate that suitable reagents can be produced by electrolyzing 20 g l −1 sodium chloride solutions at 50 ma cm −2 and 50 °c, and 3000 mg l −1 naclo 4 solutions at 5.0. Chlorine Dioxide Production.
From www.frontiersin.org
Frontiers Fresh Produce Safety and Quality Chlorine Dioxide’s Role Chlorine Dioxide Production Chlorine dioxide (clo 2) is produced from either sodium chlorite (naclo 2) or sodium chlorate (naclo 3). Chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. Results demonstrate that suitable reagents can be produced by electrolyzing 20 g l −1 sodium chloride solutions at 50 ma cm −2 and 50 °c, and 3000 mg l. Chlorine Dioxide Production.
From b2bpurchase.com
Grundfos India launches Chlorine Dioxide production system Chlorine Dioxide Production Clo 2 treatment can prevent methionine conversion, and eliminate ethylene and other substances quickly and effectively (fu and. Chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. Results demonstrate that suitable reagents can be produced by electrolyzing 20 g l −1 sodium chloride solutions at 50 ma cm −2 and 50 °c, and 3000 mg. Chlorine Dioxide Production.
From waterengineers.com.au
Chlorine Dioxide Water Engineers Chlorine Dioxide Production Chlorine dioxide (clo 2) is produced from either sodium chlorite (naclo 2) or sodium chlorate (naclo 3). Clo 2 treatment can prevent methionine conversion, and eliminate ethylene and other substances quickly and effectively (fu and. Chlorine dioxide can be efficiently produced from hydrogen peroxide and chlorate in acidic media. Production methods involve either the reduction of chlorate or the oxidation. Chlorine Dioxide Production.
From www.google.com
Patent US7452511 Reactor for production of chlorine dioxide, methods Chlorine Dioxide Production Production methods involve either the reduction of chlorate or the oxidation of chlorite. Clo 2 treatment can prevent methionine conversion, and eliminate ethylene and other substances quickly and effectively (fu and. Chlorine dioxide (clo 2) is produced from either sodium chlorite (naclo 2) or sodium chlorate (naclo 3). Both of these starting materials are relatively stable, despite their oxidizing potential.. Chlorine Dioxide Production.