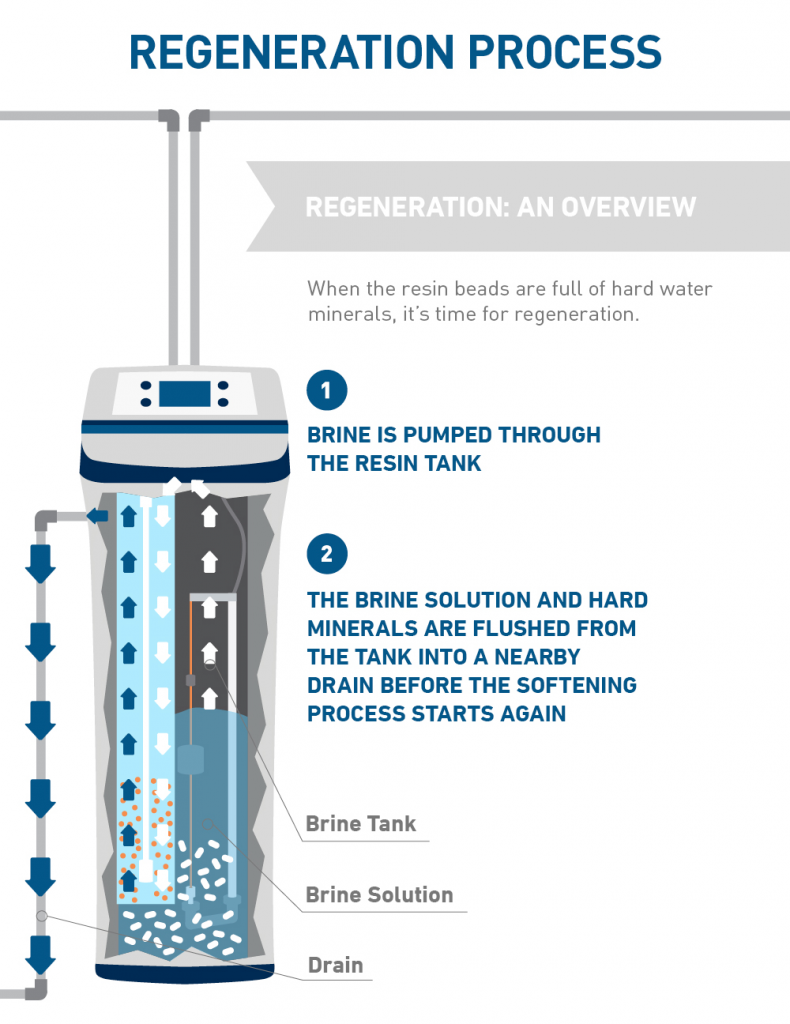
Water Softener Chemical Formula . in the softening cycle, water enters the softener and passes through the ion exchange resin charged with sodium (na+). 2 min read. water softening is achieved either by adding chemicals that form insoluble precipitates or by ion exchange. How do water softeners work? On a small scale, chemicals used for softening include ammonia, borax, calcium hydroxide (slaked lime), or trisodium phosphate, usually in conjunction with sodium carbonate (soda ash). water softeners break down salt into sodium ions and chloride and then release the polluted water into. sodium hexametaphosphate of commerce is typically a mixture of metaphosphates (empirical formula: Napo 3), of which the. Chuck wight, a chemistry professor at the university of utah,. water softener size (grains) = (water hardness (gpg) + iron content (ppm) × 5) × weekly water usage (gallons) / 0.75.
from homewater101.com
On a small scale, chemicals used for softening include ammonia, borax, calcium hydroxide (slaked lime), or trisodium phosphate, usually in conjunction with sodium carbonate (soda ash). water softeners break down salt into sodium ions and chloride and then release the polluted water into. Chuck wight, a chemistry professor at the university of utah,. in the softening cycle, water enters the softener and passes through the ion exchange resin charged with sodium (na+). How do water softeners work? water softener size (grains) = (water hardness (gpg) + iron content (ppm) × 5) × weekly water usage (gallons) / 0.75. sodium hexametaphosphate of commerce is typically a mixture of metaphosphates (empirical formula: water softening is achieved either by adding chemicals that form insoluble precipitates or by ion exchange. Napo 3), of which the. 2 min read.
How Does a Water Softener Work? Water Softening Process Diagram
Water Softener Chemical Formula water softener size (grains) = (water hardness (gpg) + iron content (ppm) × 5) × weekly water usage (gallons) / 0.75. sodium hexametaphosphate of commerce is typically a mixture of metaphosphates (empirical formula: 2 min read. water softeners break down salt into sodium ions and chloride and then release the polluted water into. in the softening cycle, water enters the softener and passes through the ion exchange resin charged with sodium (na+). On a small scale, chemicals used for softening include ammonia, borax, calcium hydroxide (slaked lime), or trisodium phosphate, usually in conjunction with sodium carbonate (soda ash). How do water softeners work? water softener size (grains) = (water hardness (gpg) + iron content (ppm) × 5) × weekly water usage (gallons) / 0.75. Napo 3), of which the. water softening is achieved either by adding chemicals that form insoluble precipitates or by ion exchange. Chuck wight, a chemistry professor at the university of utah,.
From netsolwater.com
What are the components of a water softener under O and M Plan Water Softener Chemical Formula How do water softeners work? water softeners break down salt into sodium ions and chloride and then release the polluted water into. sodium hexametaphosphate of commerce is typically a mixture of metaphosphates (empirical formula: Napo 3), of which the. water softener size (grains) = (water hardness (gpg) + iron content (ppm) × 5) × weekly water usage. Water Softener Chemical Formula.
From www.thespruce.com
All About Water Softeners and How They Work Water Softener Chemical Formula 2 min read. On a small scale, chemicals used for softening include ammonia, borax, calcium hydroxide (slaked lime), or trisodium phosphate, usually in conjunction with sodium carbonate (soda ash). Napo 3), of which the. in the softening cycle, water enters the softener and passes through the ion exchange resin charged with sodium (na+). Chuck wight, a chemistry professor. Water Softener Chemical Formula.
From watersoftenerfacts.ca
How Softeners Work Water Softener Facts Water Softener Chemical Formula How do water softeners work? Chuck wight, a chemistry professor at the university of utah,. water softener size (grains) = (water hardness (gpg) + iron content (ppm) × 5) × weekly water usage (gallons) / 0.75. 2 min read. water softeners break down salt into sodium ions and chloride and then release the polluted water into. . Water Softener Chemical Formula.
From elchoroukhost.net
Chemical Equation For Table Salt And Water Elcho Table Water Softener Chemical Formula water softener size (grains) = (water hardness (gpg) + iron content (ppm) × 5) × weekly water usage (gallons) / 0.75. in the softening cycle, water enters the softener and passes through the ion exchange resin charged with sodium (na+). How do water softeners work? water softening is achieved either by adding chemicals that form insoluble precipitates. Water Softener Chemical Formula.
From byjus.com
Describe the permutit process for softening hard water. Water Softener Chemical Formula How do water softeners work? sodium hexametaphosphate of commerce is typically a mixture of metaphosphates (empirical formula: 2 min read. Chuck wight, a chemistry professor at the university of utah,. water softener size (grains) = (water hardness (gpg) + iron content (ppm) × 5) × weekly water usage (gallons) / 0.75. On a small scale, chemicals used. Water Softener Chemical Formula.
From www.korgentech.com
Water Softeners Water Softening Plants Water Softener Chemical Formula in the softening cycle, water enters the softener and passes through the ion exchange resin charged with sodium (na+). water softener size (grains) = (water hardness (gpg) + iron content (ppm) × 5) × weekly water usage (gallons) / 0.75. sodium hexametaphosphate of commerce is typically a mixture of metaphosphates (empirical formula: How do water softeners work?. Water Softener Chemical Formula.
From www.tessshebaylo.com
Chemical Equation For Water Softening Tessshebaylo Water Softener Chemical Formula in the softening cycle, water enters the softener and passes through the ion exchange resin charged with sodium (na+). Chuck wight, a chemistry professor at the university of utah,. 2 min read. water softening is achieved either by adding chemicals that form insoluble precipitates or by ion exchange. Napo 3), of which the. How do water softeners. Water Softener Chemical Formula.
From antunes.com
Water Softener Treatments Water Softener Diagram Antunes Water Softener Chemical Formula 2 min read. sodium hexametaphosphate of commerce is typically a mixture of metaphosphates (empirical formula: water softening is achieved either by adding chemicals that form insoluble precipitates or by ion exchange. Chuck wight, a chemistry professor at the university of utah,. water softeners break down salt into sodium ions and chloride and then release the polluted. Water Softener Chemical Formula.
From www.youtube.com
LEARN AND GROW !! ION EXCHANGE PROCESS FOR WATER SOFTENING ! YouTube Water Softener Chemical Formula in the softening cycle, water enters the softener and passes through the ion exchange resin charged with sodium (na+). water softeners break down salt into sodium ions and chloride and then release the polluted water into. Napo 3), of which the. How do water softeners work? sodium hexametaphosphate of commerce is typically a mixture of metaphosphates (empirical. Water Softener Chemical Formula.
From www.watersmartsystems.com
How Does a Water Softener work? Water Experts Waterloo — WaterSmart Water Softener Chemical Formula Napo 3), of which the. water softening is achieved either by adding chemicals that form insoluble precipitates or by ion exchange. Chuck wight, a chemistry professor at the university of utah,. water softeners break down salt into sodium ions and chloride and then release the polluted water into. How do water softeners work? sodium hexametaphosphate of commerce. Water Softener Chemical Formula.
From atlas-scientific.com
Ion Exchange In Water Treatment Atlas Scientific Water Softener Chemical Formula water softening is achieved either by adding chemicals that form insoluble precipitates or by ion exchange. 2 min read. sodium hexametaphosphate of commerce is typically a mixture of metaphosphates (empirical formula: water softener size (grains) = (water hardness (gpg) + iron content (ppm) × 5) × weekly water usage (gallons) / 0.75. water softeners break. Water Softener Chemical Formula.
From purewaterblog.com
The Water Softener Regeneration Cycle Explained! Water Treatment Water Softener Chemical Formula water softener size (grains) = (water hardness (gpg) + iron content (ppm) × 5) × weekly water usage (gallons) / 0.75. Chuck wight, a chemistry professor at the university of utah,. Napo 3), of which the. in the softening cycle, water enters the softener and passes through the ion exchange resin charged with sodium (na+). sodium hexametaphosphate. Water Softener Chemical Formula.
From www.youtube.com
Ion exchange resin for water SofteningDemineralization B.Tech, BSc Water Softener Chemical Formula Napo 3), of which the. sodium hexametaphosphate of commerce is typically a mixture of metaphosphates (empirical formula: Chuck wight, a chemistry professor at the university of utah,. 2 min read. On a small scale, chemicals used for softening include ammonia, borax, calcium hydroxide (slaked lime), or trisodium phosphate, usually in conjunction with sodium carbonate (soda ash). water. Water Softener Chemical Formula.
From www.canel.my.id
Ion Exchange Water Softener Diagram Water Softener Chemical Formula On a small scale, chemicals used for softening include ammonia, borax, calcium hydroxide (slaked lime), or trisodium phosphate, usually in conjunction with sodium carbonate (soda ash). Chuck wight, a chemistry professor at the university of utah,. sodium hexametaphosphate of commerce is typically a mixture of metaphosphates (empirical formula: Napo 3), of which the. How do water softeners work? . Water Softener Chemical Formula.
From purewaterblog.com
Citric Acid Water Softeners Everything You Need to Know Water Treatment Water Softener Chemical Formula in the softening cycle, water enters the softener and passes through the ion exchange resin charged with sodium (na+). water softening is achieved either by adding chemicals that form insoluble precipitates or by ion exchange. How do water softeners work? water softener size (grains) = (water hardness (gpg) + iron content (ppm) × 5) × weekly water. Water Softener Chemical Formula.
From discover.hubpages.com
The Chemistry of LimeSoda Ash Precipitation Water Softening HubPages Water Softener Chemical Formula How do water softeners work? Chuck wight, a chemistry professor at the university of utah,. in the softening cycle, water enters the softener and passes through the ion exchange resin charged with sodium (na+). water softener size (grains) = (water hardness (gpg) + iron content (ppm) × 5) × weekly water usage (gallons) / 0.75. water softening. Water Softener Chemical Formula.
From www.cleanwaterstore.com
Well Water Diagram Softener Water Softener Chemical Formula Napo 3), of which the. in the softening cycle, water enters the softener and passes through the ion exchange resin charged with sodium (na+). On a small scale, chemicals used for softening include ammonia, borax, calcium hydroxide (slaked lime), or trisodium phosphate, usually in conjunction with sodium carbonate (soda ash). How do water softeners work? water softener size. Water Softener Chemical Formula.
From waterseer.org
Water Conditioner Vs Water Softener What's The Difference? Water Softener Chemical Formula in the softening cycle, water enters the softener and passes through the ion exchange resin charged with sodium (na+). Chuck wight, a chemistry professor at the university of utah,. water softening is achieved either by adding chemicals that form insoluble precipitates or by ion exchange. 2 min read. Napo 3), of which the. water softeners break. Water Softener Chemical Formula.
From watervex.com
Water Softener Chemical Formula Secrets Unveiled Water Softener Chemical Formula water softeners break down salt into sodium ions and chloride and then release the polluted water into. Napo 3), of which the. 2 min read. in the softening cycle, water enters the softener and passes through the ion exchange resin charged with sodium (na+). water softening is achieved either by adding chemicals that form insoluble precipitates. Water Softener Chemical Formula.
From www.reverseosmosis.com
Chemicals for Water Treatment Water Softener Cleaners Water Softener Chemical Formula Chuck wight, a chemistry professor at the university of utah,. water softening is achieved either by adding chemicals that form insoluble precipitates or by ion exchange. sodium hexametaphosphate of commerce is typically a mixture of metaphosphates (empirical formula: 2 min read. water softeners break down salt into sodium ions and chloride and then release the polluted. Water Softener Chemical Formula.
From waterseer.org
6 Common Water Softener Problems (+ How to Fix Them) Water Softener Chemical Formula water softener size (grains) = (water hardness (gpg) + iron content (ppm) × 5) × weekly water usage (gallons) / 0.75. Napo 3), of which the. water softening is achieved either by adding chemicals that form insoluble precipitates or by ion exchange. water softeners break down salt into sodium ions and chloride and then release the polluted. Water Softener Chemical Formula.
From netsolwater.com
How is the resin capacity calculated in a water softener Water Softener Chemical Formula How do water softeners work? On a small scale, chemicals used for softening include ammonia, borax, calcium hydroxide (slaked lime), or trisodium phosphate, usually in conjunction with sodium carbonate (soda ash). water softeners break down salt into sodium ions and chloride and then release the polluted water into. in the softening cycle, water enters the softener and passes. Water Softener Chemical Formula.
From issuu.com
How Does Water Softener Work Chemistry? by AmericanLifeguardManual Issuu Water Softener Chemical Formula in the softening cycle, water enters the softener and passes through the ion exchange resin charged with sodium (na+). Napo 3), of which the. sodium hexametaphosphate of commerce is typically a mixture of metaphosphates (empirical formula: On a small scale, chemicals used for softening include ammonia, borax, calcium hydroxide (slaked lime), or trisodium phosphate, usually in conjunction with. Water Softener Chemical Formula.
From www.indiamart.com
Water Softener Chemical, for Water Softening, Packaging Type Plastic Water Softener Chemical Formula Chuck wight, a chemistry professor at the university of utah,. On a small scale, chemicals used for softening include ammonia, borax, calcium hydroxide (slaked lime), or trisodium phosphate, usually in conjunction with sodium carbonate (soda ash). water softeners break down salt into sodium ions and chloride and then release the polluted water into. in the softening cycle, water. Water Softener Chemical Formula.
From waterseer.org
Water Conditioner Vs Water Softener What's The Difference? Water Softener Chemical Formula On a small scale, chemicals used for softening include ammonia, borax, calcium hydroxide (slaked lime), or trisodium phosphate, usually in conjunction with sodium carbonate (soda ash). How do water softeners work? sodium hexametaphosphate of commerce is typically a mixture of metaphosphates (empirical formula: Napo 3), of which the. water softeners break down salt into sodium ions and chloride. Water Softener Chemical Formula.
From homewater101.com
How Does a Water Softener Work? Water Softening Process Diagram Water Softener Chemical Formula in the softening cycle, water enters the softener and passes through the ion exchange resin charged with sodium (na+). Chuck wight, a chemistry professor at the university of utah,. water softening is achieved either by adding chemicals that form insoluble precipitates or by ion exchange. water softener size (grains) = (water hardness (gpg) + iron content (ppm). Water Softener Chemical Formula.
From www.indiamart.com
Water Softener Chemical at Rs 88/kilogram Water Softener Chemical in Water Softener Chemical Formula Napo 3), of which the. water softener size (grains) = (water hardness (gpg) + iron content (ppm) × 5) × weekly water usage (gallons) / 0.75. 2 min read. in the softening cycle, water enters the softener and passes through the ion exchange resin charged with sodium (na+). water softening is achieved either by adding chemicals. Water Softener Chemical Formula.
From www.youtube.com
Water Softener Plant Operation I Softener water system I How does a Water Softener Chemical Formula Chuck wight, a chemistry professor at the university of utah,. 2 min read. water softening is achieved either by adding chemicals that form insoluble precipitates or by ion exchange. water softeners break down salt into sodium ions and chloride and then release the polluted water into. Napo 3), of which the. How do water softeners work? . Water Softener Chemical Formula.
From www.korgentech.com
Water Softeners Water Softening Plants Water Softener Chemical Formula in the softening cycle, water enters the softener and passes through the ion exchange resin charged with sodium (na+). water softener size (grains) = (water hardness (gpg) + iron content (ppm) × 5) × weekly water usage (gallons) / 0.75. sodium hexametaphosphate of commerce is typically a mixture of metaphosphates (empirical formula: Napo 3), of which the.. Water Softener Chemical Formula.
From homewater101.com
How Does a Water Softener Work? Water Softening Process Diagram Water Softener Chemical Formula sodium hexametaphosphate of commerce is typically a mixture of metaphosphates (empirical formula: in the softening cycle, water enters the softener and passes through the ion exchange resin charged with sodium (na+). 2 min read. water softeners break down salt into sodium ions and chloride and then release the polluted water into. water softening is achieved. Water Softener Chemical Formula.
From tinyhousegarage.com
How Does Water Softener Work Chemistry? Water Softener Chemical Formula sodium hexametaphosphate of commerce is typically a mixture of metaphosphates (empirical formula: On a small scale, chemicals used for softening include ammonia, borax, calcium hydroxide (slaked lime), or trisodium phosphate, usually in conjunction with sodium carbonate (soda ash). water softeners break down salt into sodium ions and chloride and then release the polluted water into. in the. Water Softener Chemical Formula.
From complete-water.com
Industrial Water Softener Parts, Benefits, CWS Water Softener Chemical Formula Chuck wight, a chemistry professor at the university of utah,. 2 min read. water softener size (grains) = (water hardness (gpg) + iron content (ppm) × 5) × weekly water usage (gallons) / 0.75. water softening is achieved either by adding chemicals that form insoluble precipitates or by ion exchange. in the softening cycle, water enters. Water Softener Chemical Formula.
From waterfilterguru.com
How to Remove Salt from Softened Water (Top 3 Methods) Water Softener Chemical Formula Napo 3), of which the. On a small scale, chemicals used for softening include ammonia, borax, calcium hydroxide (slaked lime), or trisodium phosphate, usually in conjunction with sodium carbonate (soda ash). sodium hexametaphosphate of commerce is typically a mixture of metaphosphates (empirical formula: in the softening cycle, water enters the softener and passes through the ion exchange resin. Water Softener Chemical Formula.
From netsolwater.com
How to add sodium and potassium in Water with help of Water Softener Water Softener Chemical Formula Chuck wight, a chemistry professor at the university of utah,. sodium hexametaphosphate of commerce is typically a mixture of metaphosphates (empirical formula: How do water softeners work? On a small scale, chemicals used for softening include ammonia, borax, calcium hydroxide (slaked lime), or trisodium phosphate, usually in conjunction with sodium carbonate (soda ash). water softening is achieved either. Water Softener Chemical Formula.
From www.ecomfort.com
Water Softener Buyers Guide How to Pick the Best Water Softener Water Softener Chemical Formula sodium hexametaphosphate of commerce is typically a mixture of metaphosphates (empirical formula: water softening is achieved either by adding chemicals that form insoluble precipitates or by ion exchange. Napo 3), of which the. in the softening cycle, water enters the softener and passes through the ion exchange resin charged with sodium (na+). Chuck wight, a chemistry professor. Water Softener Chemical Formula.