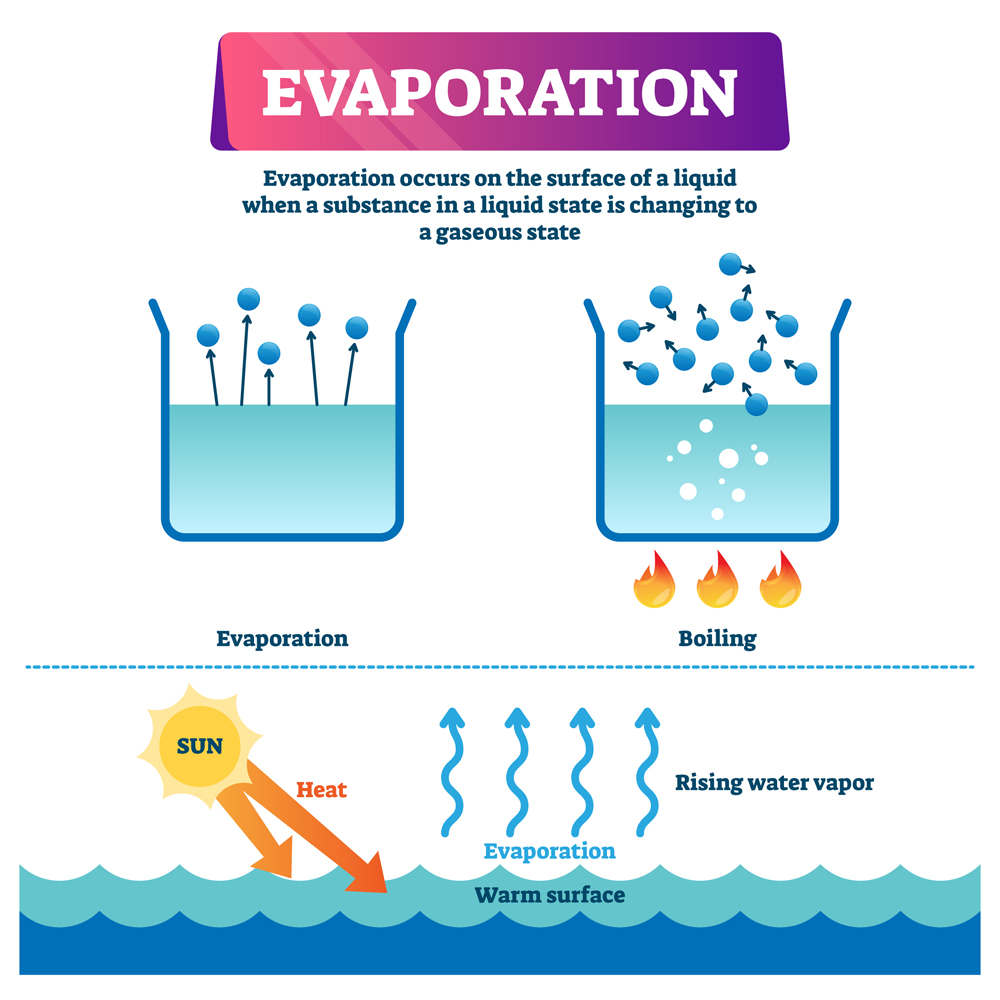
Why Can Water Evaporate At Room Temperature . — boiling point of water is 100 degree celsius. — areas with high temperatures and large bodies of water, such as tropical islands and swamps, are usually very. — even at low temperatures, there are some water molecules are have enough energy to escape and that's why evaporation in water can. As water is heated, its vapour pressure overcomes ambient air pressure at a lower temperature i.e. — if water evaporates at room temperature because a small percentage of the molecules have enough energy to. — water evaporates at room temperature because a portion of the water. — water evaporates at room temperature because the molecules at the. at higher elevations, air pressure is lower; — water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in. The temperature at which water in liquid form is converted into gaseous form.
from www.scienceabc.com
— boiling point of water is 100 degree celsius. The temperature at which water in liquid form is converted into gaseous form. — even at low temperatures, there are some water molecules are have enough energy to escape and that's why evaporation in water can. — water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in. — areas with high temperatures and large bodies of water, such as tropical islands and swamps, are usually very. — water evaporates at room temperature because the molecules at the. — if water evaporates at room temperature because a small percentage of the molecules have enough energy to. As water is heated, its vapour pressure overcomes ambient air pressure at a lower temperature i.e. at higher elevations, air pressure is lower; — water evaporates at room temperature because a portion of the water.
Why Does Water Evaporate At Room Temperature?
Why Can Water Evaporate At Room Temperature — areas with high temperatures and large bodies of water, such as tropical islands and swamps, are usually very. As water is heated, its vapour pressure overcomes ambient air pressure at a lower temperature i.e. — water evaporates at room temperature because a portion of the water. The temperature at which water in liquid form is converted into gaseous form. — even at low temperatures, there are some water molecules are have enough energy to escape and that's why evaporation in water can. — boiling point of water is 100 degree celsius. — areas with high temperatures and large bodies of water, such as tropical islands and swamps, are usually very. — if water evaporates at room temperature because a small percentage of the molecules have enough energy to. at higher elevations, air pressure is lower; — water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in. — water evaporates at room temperature because the molecules at the.
From dxowlbddi.blob.core.windows.net
Explain The Evaporation Process at James Ligon blog Why Can Water Evaporate At Room Temperature — water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in. — if water evaporates at room temperature because a small percentage of the molecules have enough energy to. — water evaporates at room temperature because a portion of the water. at higher elevations, air pressure. Why Can Water Evaporate At Room Temperature.
From www.teachoo.com
Why is Water liquid at room temperature? Give reasons Teachoo Why Can Water Evaporate At Room Temperature — boiling point of water is 100 degree celsius. The temperature at which water in liquid form is converted into gaseous form. — water evaporates at room temperature because the molecules at the. — water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in. — if. Why Can Water Evaporate At Room Temperature.
From www.youtube.com
Why does water evaporate at room temperature? YouTube Why Can Water Evaporate At Room Temperature at higher elevations, air pressure is lower; — even at low temperatures, there are some water molecules are have enough energy to escape and that's why evaporation in water can. As water is heated, its vapour pressure overcomes ambient air pressure at a lower temperature i.e. — boiling point of water is 100 degree celsius. The temperature. Why Can Water Evaporate At Room Temperature.
From bestroom.one
Why Does Water Evaporate At Room Temperature bestroom.one Why Can Water Evaporate At Room Temperature The temperature at which water in liquid form is converted into gaseous form. — areas with high temperatures and large bodies of water, such as tropical islands and swamps, are usually very. — if water evaporates at room temperature because a small percentage of the molecules have enough energy to. As water is heated, its vapour pressure overcomes. Why Can Water Evaporate At Room Temperature.
From www.youtube.com
Why Does Water Evaporate at Room Temperature? YouTube Why Can Water Evaporate At Room Temperature — boiling point of water is 100 degree celsius. — if water evaporates at room temperature because a small percentage of the molecules have enough energy to. at higher elevations, air pressure is lower; — water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in. . Why Can Water Evaporate At Room Temperature.
From diagramlibraryflew.z13.web.core.windows.net
Diagram Of Evaporation Why Can Water Evaporate At Room Temperature — even at low temperatures, there are some water molecules are have enough energy to escape and that's why evaporation in water can. As water is heated, its vapour pressure overcomes ambient air pressure at a lower temperature i.e. — areas with high temperatures and large bodies of water, such as tropical islands and swamps, are usually very.. Why Can Water Evaporate At Room Temperature.
From www.amazon.ca
Why Does Water Evaporate? All about Heat and Temperature Moore, Rob Why Can Water Evaporate At Room Temperature — areas with high temperatures and large bodies of water, such as tropical islands and swamps, are usually very. — water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in. — water evaporates at room temperature because a portion of the water. As water is heated, its. Why Can Water Evaporate At Room Temperature.
From www.youtube.com
What elements are liquid at room temperature? YouTube Why Can Water Evaporate At Room Temperature — water evaporates at room temperature because the molecules at the. — even at low temperatures, there are some water molecules are have enough energy to escape and that's why evaporation in water can. — water evaporates at room temperature because a portion of the water. As water is heated, its vapour pressure overcomes ambient air pressure. Why Can Water Evaporate At Room Temperature.
From science.howstuffworks.com
1 Why does water evaporate at room temperature? 10 Science Questions Why Can Water Evaporate At Room Temperature at higher elevations, air pressure is lower; — if water evaporates at room temperature because a small percentage of the molecules have enough energy to. — water evaporates at room temperature because the molecules at the. — water evaporates at room temperature because a portion of the water. As water is heated, its vapour pressure overcomes. Why Can Water Evaporate At Room Temperature.
From www.numerade.com
SOLVEDWhy does water spilled on the floor evaporate even though ΔG^∘ Why Can Water Evaporate At Room Temperature — if water evaporates at room temperature because a small percentage of the molecules have enough energy to. The temperature at which water in liquid form is converted into gaseous form. — even at low temperatures, there are some water molecules are have enough energy to escape and that's why evaporation in water can. — boiling point. Why Can Water Evaporate At Room Temperature.
From exoenxpbj.blob.core.windows.net
What Evaporates Quicker Water Or Alcohol at Dolores Boren blog Why Can Water Evaporate At Room Temperature As water is heated, its vapour pressure overcomes ambient air pressure at a lower temperature i.e. — even at low temperatures, there are some water molecules are have enough energy to escape and that's why evaporation in water can. — water evaporates at room temperature because the molecules at the. — water evaporates at room temperature because. Why Can Water Evaporate At Room Temperature.
From poe.com
What is the duration required for 1 cup of water to evaporate at room Why Can Water Evaporate At Room Temperature at higher elevations, air pressure is lower; — water evaporates at room temperature because the molecules at the. As water is heated, its vapour pressure overcomes ambient air pressure at a lower temperature i.e. — if water evaporates at room temperature because a small percentage of the molecules have enough energy to. — even at low. Why Can Water Evaporate At Room Temperature.
From www.youtube.com
How does water evaporate at room temperaturevaporization and Why Can Water Evaporate At Room Temperature — areas with high temperatures and large bodies of water, such as tropical islands and swamps, are usually very. — water evaporates at room temperature because a portion of the water. — even at low temperatures, there are some water molecules are have enough energy to escape and that's why evaporation in water can. — boiling. Why Can Water Evaporate At Room Temperature.
From www.slideserve.com
PPT Warmup PowerPoint Presentation, free download ID230026 Why Can Water Evaporate At Room Temperature The temperature at which water in liquid form is converted into gaseous form. — if water evaporates at room temperature because a small percentage of the molecules have enough energy to. — water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in. at higher elevations, air pressure. Why Can Water Evaporate At Room Temperature.
From www.scienceabc.com
Why Does Water Evaporate At Room Temperature? Why Can Water Evaporate At Room Temperature at higher elevations, air pressure is lower; — boiling point of water is 100 degree celsius. As water is heated, its vapour pressure overcomes ambient air pressure at a lower temperature i.e. — if water evaporates at room temperature because a small percentage of the molecules have enough energy to. — water evaporates at room temperature. Why Can Water Evaporate At Room Temperature.
From 9to5science.com
[Solved] Why can't I evaporate water without wind, just 9to5Science Why Can Water Evaporate At Room Temperature — if water evaporates at room temperature because a small percentage of the molecules have enough energy to. — water evaporates at room temperature because a portion of the water. As water is heated, its vapour pressure overcomes ambient air pressure at a lower temperature i.e. — boiling point of water is 100 degree celsius. —. Why Can Water Evaporate At Room Temperature.
From physics.stackexchange.com
thermodynamics Evaporation of water at room temperature Physics Why Can Water Evaporate At Room Temperature — water evaporates at room temperature because the molecules at the. at higher elevations, air pressure is lower; As water is heated, its vapour pressure overcomes ambient air pressure at a lower temperature i.e. The temperature at which water in liquid form is converted into gaseous form. — areas with high temperatures and large bodies of water,. Why Can Water Evaporate At Room Temperature.
From www.numerade.com
SOLVED Some quantity of water at room temperature is placed in an open Why Can Water Evaporate At Room Temperature — areas with high temperatures and large bodies of water, such as tropical islands and swamps, are usually very. — boiling point of water is 100 degree celsius. As water is heated, its vapour pressure overcomes ambient air pressure at a lower temperature i.e. — water evaporates at room temperature because the molecules at the surface of. Why Can Water Evaporate At Room Temperature.
From 9to5science.com
[Solved] Why does water evaporate at room temperature? 9to5Science Why Can Water Evaporate At Room Temperature — water evaporates at room temperature because the molecules at the. — boiling point of water is 100 degree celsius. at higher elevations, air pressure is lower; — water evaporates at room temperature because a portion of the water. — even at low temperatures, there are some water molecules are have enough energy to escape. Why Can Water Evaporate At Room Temperature.
From www.youtube.com
Why does water evaporate at room temp? YouTube Why Can Water Evaporate At Room Temperature — water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in. As water is heated, its vapour pressure overcomes ambient air pressure at a lower temperature i.e. The temperature at which water in liquid form is converted into gaseous form. — boiling point of water is 100 degree. Why Can Water Evaporate At Room Temperature.
From www.youtube.com
Can water evaporate at 10 degree Celsius? YouTube Why Can Water Evaporate At Room Temperature — water evaporates at room temperature because the molecules at the. — areas with high temperatures and large bodies of water, such as tropical islands and swamps, are usually very. — water evaporates at room temperature because a portion of the water. — even at low temperatures, there are some water molecules are have enough energy. Why Can Water Evaporate At Room Temperature.
From www.studocu.com
Phase Changes & Vapor Pressure Why Does Water Evaporate at Room Temp Why Can Water Evaporate At Room Temperature — water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in. As water is heated, its vapour pressure overcomes ambient air pressure at a lower temperature i.e. — even at low temperatures, there are some water molecules are have enough energy to escape and that's why evaporation in. Why Can Water Evaporate At Room Temperature.
From www.slideserve.com
PPT Implication of Intermolecular Forces PowerPoint Presentation Why Can Water Evaporate At Room Temperature — if water evaporates at room temperature because a small percentage of the molecules have enough energy to. As water is heated, its vapour pressure overcomes ambient air pressure at a lower temperature i.e. — areas with high temperatures and large bodies of water, such as tropical islands and swamps, are usually very. — water evaporates at. Why Can Water Evaporate At Room Temperature.
From www.scienceabc.com
Why Does Water Evaporate At Room Temperature? Why Can Water Evaporate At Room Temperature at higher elevations, air pressure is lower; The temperature at which water in liquid form is converted into gaseous form. — water evaporates at room temperature because the molecules at the. — areas with high temperatures and large bodies of water, such as tropical islands and swamps, are usually very. — if water evaporates at room. Why Can Water Evaporate At Room Temperature.
From slideplayer.com
108 Entropy, Free Energy, and Spontaneity (Section 10.10) ppt download Why Can Water Evaporate At Room Temperature at higher elevations, air pressure is lower; As water is heated, its vapour pressure overcomes ambient air pressure at a lower temperature i.e. — even at low temperatures, there are some water molecules are have enough energy to escape and that's why evaporation in water can. — water evaporates at room temperature because the molecules at the.. Why Can Water Evaporate At Room Temperature.
From www.vroble.com
Why Does Water Evaporate at Room Temperature? Why Can Water Evaporate At Room Temperature — if water evaporates at room temperature because a small percentage of the molecules have enough energy to. — boiling point of water is 100 degree celsius. — water evaporates at room temperature because a portion of the water. — water evaporates at room temperature because the molecules at the. The temperature at which water in. Why Can Water Evaporate At Room Temperature.
From exostggiu.blob.core.windows.net
Can Water Evaporate In The Dark at Pamela Fifield blog Why Can Water Evaporate At Room Temperature — even at low temperatures, there are some water molecules are have enough energy to escape and that's why evaporation in water can. — areas with high temperatures and large bodies of water, such as tropical islands and swamps, are usually very. The temperature at which water in liquid form is converted into gaseous form. — boiling. Why Can Water Evaporate At Room Temperature.
From www.numerade.com
SOLVEDIf you place water at room temperature in a wellinsulated cup Why Can Water Evaporate At Room Temperature — water evaporates at room temperature because a portion of the water. As water is heated, its vapour pressure overcomes ambient air pressure at a lower temperature i.e. — if water evaporates at room temperature because a small percentage of the molecules have enough energy to. — boiling point of water is 100 degree celsius. The temperature. Why Can Water Evaporate At Room Temperature.
From www.scienceabc.com
Why Does Water Evaporate At Room Temperature? Why Can Water Evaporate At Room Temperature — water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in. — water evaporates at room temperature because a portion of the water. — boiling point of water is 100 degree celsius. The temperature at which water in liquid form is converted into gaseous form. —. Why Can Water Evaporate At Room Temperature.
From tipseri.com
How long does it take for water to evaporate at room temperature? Tipseri Why Can Water Evaporate At Room Temperature As water is heated, its vapour pressure overcomes ambient air pressure at a lower temperature i.e. — even at low temperatures, there are some water molecules are have enough energy to escape and that's why evaporation in water can. at higher elevations, air pressure is lower; — areas with high temperatures and large bodies of water, such. Why Can Water Evaporate At Room Temperature.
From sciencenotes.org
How to Boil Water at Room Temperature Why Can Water Evaporate At Room Temperature As water is heated, its vapour pressure overcomes ambient air pressure at a lower temperature i.e. — even at low temperatures, there are some water molecules are have enough energy to escape and that's why evaporation in water can. — water evaporates at room temperature because a portion of the water. — if water evaporates at room. Why Can Water Evaporate At Room Temperature.
From mavink.com
Water Evaporation Chart Why Can Water Evaporate At Room Temperature As water is heated, its vapour pressure overcomes ambient air pressure at a lower temperature i.e. — if water evaporates at room temperature because a small percentage of the molecules have enough energy to. — water evaporates at room temperature because the molecules at the. — even at low temperatures, there are some water molecules are have. Why Can Water Evaporate At Room Temperature.
From monica-well-sims.blogspot.com
A Substance That Evaporates at Room Temperature Is Described as Why Can Water Evaporate At Room Temperature — boiling point of water is 100 degree celsius. — water evaporates at room temperature because a portion of the water. — even at low temperatures, there are some water molecules are have enough energy to escape and that's why evaporation in water can. — water evaporates at room temperature because the molecules at the surface. Why Can Water Evaporate At Room Temperature.
From www.vrogue.co
Why Does Water Vapor Form When Water Is Heated To Its vrogue.co Why Can Water Evaporate At Room Temperature The temperature at which water in liquid form is converted into gaseous form. at higher elevations, air pressure is lower; — areas with high temperatures and large bodies of water, such as tropical islands and swamps, are usually very. As water is heated, its vapour pressure overcomes ambient air pressure at a lower temperature i.e. — even. Why Can Water Evaporate At Room Temperature.
From young6science3.weebly.com
5Th grade science The Water Cycle Why Can Water Evaporate At Room Temperature — water evaporates at room temperature because the molecules at the. — water evaporates at room temperature because a portion of the water. The temperature at which water in liquid form is converted into gaseous form. — water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in.. Why Can Water Evaporate At Room Temperature.