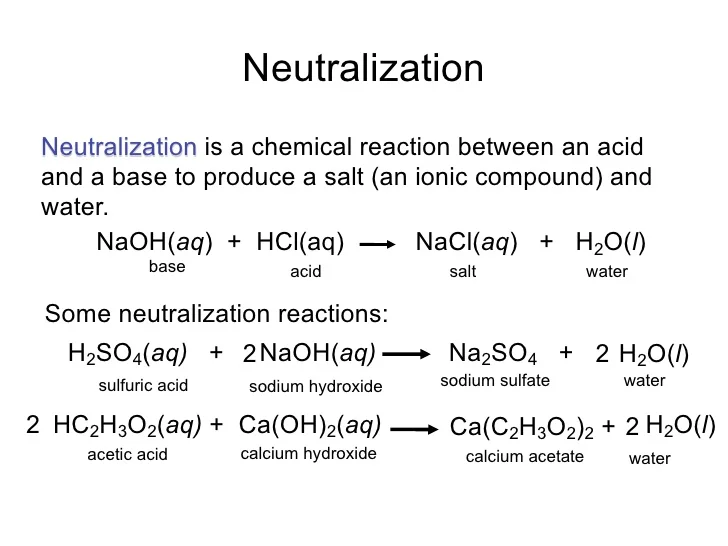
What Reacts With Sodium Hydroxide . Sodium hydroxide is a strong base, meaning it reacts vigorously with acids to form water and a salt. The reaction between sodium hydroxide and hydrochloric acid can be shown in the following equation: Naoh + hcl → nacl + h2o. This type of reaction is known as a neutralization reaction. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Oil and fat triglycerides react with sodium hydroxide to form fatty acid glycerol and sodium salts, the latter being the soaps. In a similar way, sulphuric acid (h 2 so 4). The extraction of excess sodium hydroxide is done with great. The aqueous solution of sodium hydroxide engages in a chemical reaction with solutions containing ferrous and ferric salts, leading to the creation of a visually cloudy. So i wanted to know what the reaction between sodium hydroxide and carbon dioxide can be, and upon research i got 2 answers. 7 rows are precipitation reactions. \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +.
from www.vrogue.co
7 rows are precipitation reactions. So i wanted to know what the reaction between sodium hydroxide and carbon dioxide can be, and upon research i got 2 answers. The extraction of excess sodium hydroxide is done with great. This type of reaction is known as a neutralization reaction. The reaction between sodium hydroxide and hydrochloric acid can be shown in the following equation: For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +. Sodium hydroxide is a strong base, meaning it reacts vigorously with acids to form water and a salt. The aqueous solution of sodium hydroxide engages in a chemical reaction with solutions containing ferrous and ferric salts, leading to the creation of a visually cloudy. Naoh + hcl → nacl + h2o.
An Aqueous Solution Of Sodium Hydroxide Reacts Comple vrogue.co
What Reacts With Sodium Hydroxide For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. 7 rows are precipitation reactions. The extraction of excess sodium hydroxide is done with great. Oil and fat triglycerides react with sodium hydroxide to form fatty acid glycerol and sodium salts, the latter being the soaps. \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +. The aqueous solution of sodium hydroxide engages in a chemical reaction with solutions containing ferrous and ferric salts, leading to the creation of a visually cloudy. Naoh + hcl → nacl + h2o. So i wanted to know what the reaction between sodium hydroxide and carbon dioxide can be, and upon research i got 2 answers. The reaction between sodium hydroxide and hydrochloric acid can be shown in the following equation: In a similar way, sulphuric acid (h 2 so 4). Sodium hydroxide is a strong base, meaning it reacts vigorously with acids to form water and a salt. This type of reaction is known as a neutralization reaction.
From www.youtube.com
Reaction of Sodium Hydroxide with Metals, Chemistry Lecture Sabaq.pk YouTube What Reacts With Sodium Hydroxide Sodium hydroxide is a strong base, meaning it reacts vigorously with acids to form water and a salt. This type of reaction is known as a neutralization reaction. So i wanted to know what the reaction between sodium hydroxide and carbon dioxide can be, and upon research i got 2 answers. In a similar way, sulphuric acid (h 2 so. What Reacts With Sodium Hydroxide.
From www.gkseries.com
What is formed when zinc reacts with sodium hydroxide? What Reacts With Sodium Hydroxide In a similar way, sulphuric acid (h 2 so 4). The reaction between sodium hydroxide and hydrochloric acid can be shown in the following equation: Naoh + hcl → nacl + h2o. Oil and fat triglycerides react with sodium hydroxide to form fatty acid glycerol and sodium salts, the latter being the soaps. 7 rows are precipitation reactions. The extraction. What Reacts With Sodium Hydroxide.
From www.sciencephoto.com
Sodium Hydroxide Reacts with Hydrochloric Acid Stock Image C036/3415 Science Photo Library What Reacts With Sodium Hydroxide So i wanted to know what the reaction between sodium hydroxide and carbon dioxide can be, and upon research i got 2 answers. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. The extraction of excess sodium hydroxide is done with great. This type of reaction is known as a neutralization reaction. Sodium hydroxide is. What Reacts With Sodium Hydroxide.
From www.numerade.com
SOLVED write balanced chemical equation between oxalic acid and sodium hydroxide reaction What Reacts With Sodium Hydroxide In a similar way, sulphuric acid (h 2 so 4). \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +. Naoh + hcl → nacl + h2o. 7 rows are precipitation reactions. This type of reaction is known as a neutralization reaction. The aqueous solution of sodium hydroxide engages in a chemical reaction with solutions containing ferrous and ferric salts, leading to the creation. What Reacts With Sodium Hydroxide.
From www.youtube.com
How to Balance NaOH + Al = Al(OH)3 + Na (Sodium Hydroxide and Aluminum) YouTube What Reacts With Sodium Hydroxide The aqueous solution of sodium hydroxide engages in a chemical reaction with solutions containing ferrous and ferric salts, leading to the creation of a visually cloudy. The extraction of excess sodium hydroxide is done with great. This type of reaction is known as a neutralization reaction. Sodium hydroxide is a strong base, meaning it reacts vigorously with acids to form. What Reacts With Sodium Hydroxide.
From basdemax.netlify.app
28++ Hydrochloric Acid And Sodium Hydroxide Balanced Equation Basdemax What Reacts With Sodium Hydroxide In a similar way, sulphuric acid (h 2 so 4). Sodium hydroxide is a strong base, meaning it reacts vigorously with acids to form water and a salt. \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +. The extraction of excess sodium hydroxide is done with great. The reaction between sodium hydroxide and hydrochloric acid can be shown in the following equation: For. What Reacts With Sodium Hydroxide.
From www.youtube.com
What happens when Sodium Hydroxide and Aluminium Reacts..? Exothermic Reaction YouTube What Reacts With Sodium Hydroxide 7 rows are precipitation reactions. The aqueous solution of sodium hydroxide engages in a chemical reaction with solutions containing ferrous and ferric salts, leading to the creation of a visually cloudy. Oil and fat triglycerides react with sodium hydroxide to form fatty acid glycerol and sodium salts, the latter being the soaps. The reaction between sodium hydroxide and hydrochloric acid. What Reacts With Sodium Hydroxide.
From www.chegg.com
Solved a Balance the reaction of sodium hydroxide and What Reacts With Sodium Hydroxide In a similar way, sulphuric acid (h 2 so 4). The reaction between sodium hydroxide and hydrochloric acid can be shown in the following equation: The extraction of excess sodium hydroxide is done with great. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. Sodium hydroxide is a strong base, meaning it reacts vigorously with. What Reacts With Sodium Hydroxide.
From chemicaldb.netlify.app
What substances does sodium hydroxide react with What Reacts With Sodium Hydroxide \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +. Sodium hydroxide is a strong base, meaning it reacts vigorously with acids to form water and a salt. The aqueous solution of sodium hydroxide engages in a chemical reaction with solutions containing ferrous and ferric salts, leading to the creation of a visually cloudy. 7 rows are precipitation reactions. Oil and fat triglycerides react. What Reacts With Sodium Hydroxide.
From www.alamy.com
Sodium Reacting with Water Generating Sodium Hydroxide and Hydrogen which Ignites Stock Photo What Reacts With Sodium Hydroxide Naoh + hcl → nacl + h2o. The reaction between sodium hydroxide and hydrochloric acid can be shown in the following equation: In a similar way, sulphuric acid (h 2 so 4). The extraction of excess sodium hydroxide is done with great. \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +. The aqueous solution of sodium hydroxide engages in a chemical reaction with. What Reacts With Sodium Hydroxide.
From stock.adobe.com
Double displacement reaction sodium hydroxide and copper sulfate. Types of chemical reactions What Reacts With Sodium Hydroxide The reaction between sodium hydroxide and hydrochloric acid can be shown in the following equation: For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +. In a similar way, sulphuric acid (h 2 so 4). Oil and fat triglycerides react with sodium hydroxide to form fatty acid glycerol and sodium. What Reacts With Sodium Hydroxide.
From www.vrogue.co
An Aqueous Solution Of Sodium Hydroxide Reacts Comple vrogue.co What Reacts With Sodium Hydroxide \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +. 7 rows are precipitation reactions. Sodium hydroxide is a strong base, meaning it reacts vigorously with acids to form water and a salt. So i wanted to know what the reaction between sodium hydroxide and carbon dioxide can be, and upon research i got 2 answers. This type of reaction is known as a. What Reacts With Sodium Hydroxide.
From www.numerade.com
SOLVED Sodium metal reacts with water to produce hydrogen gas and sodium hydroxide according to What Reacts With Sodium Hydroxide So i wanted to know what the reaction between sodium hydroxide and carbon dioxide can be, and upon research i got 2 answers. 7 rows are precipitation reactions. \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +. Naoh + hcl → nacl + h2o. In a similar way, sulphuric acid (h 2 so 4). The aqueous solution of sodium hydroxide engages in a. What Reacts With Sodium Hydroxide.
From www.vrogue.co
An Aqueous Solution Of Sodium Hydroxide Reacts Comple vrogue.co What Reacts With Sodium Hydroxide For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +. Oil and fat triglycerides react with sodium hydroxide to form fatty acid glycerol and sodium salts, the latter being the soaps. So i wanted to know what the reaction between sodium hydroxide and carbon dioxide can be, and upon research. What Reacts With Sodium Hydroxide.
From www.animalia-life.club
Sodium Hydroxide Structure What Reacts With Sodium Hydroxide This type of reaction is known as a neutralization reaction. So i wanted to know what the reaction between sodium hydroxide and carbon dioxide can be, and upon research i got 2 answers. The extraction of excess sodium hydroxide is done with great. 7 rows are precipitation reactions. \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +. Naoh + hcl → nacl +. What Reacts With Sodium Hydroxide.
From www.nagwa.com
Question Video Writing the Balanced Net Ionic Equation for the Reaction between the Ammonium What Reacts With Sodium Hydroxide Oil and fat triglycerides react with sodium hydroxide to form fatty acid glycerol and sodium salts, the latter being the soaps. So i wanted to know what the reaction between sodium hydroxide and carbon dioxide can be, and upon research i got 2 answers. The aqueous solution of sodium hydroxide engages in a chemical reaction with solutions containing ferrous and. What Reacts With Sodium Hydroxide.
From www.coursehero.com
Solved Chemical Equations Solid sodium metal reacts with water, giving a solution of sodium What Reacts With Sodium Hydroxide The extraction of excess sodium hydroxide is done with great. Sodium hydroxide is a strong base, meaning it reacts vigorously with acids to form water and a salt. The aqueous solution of sodium hydroxide engages in a chemical reaction with solutions containing ferrous and ferric salts, leading to the creation of a visually cloudy. 7 rows are precipitation reactions. \[\ce{zn(s). What Reacts With Sodium Hydroxide.
From www.vrogue.co
An Aqueous Solution Of Sodium Hydroxide Reacts Comple vrogue.co What Reacts With Sodium Hydroxide So i wanted to know what the reaction between sodium hydroxide and carbon dioxide can be, and upon research i got 2 answers. Sodium hydroxide is a strong base, meaning it reacts vigorously with acids to form water and a salt. The aqueous solution of sodium hydroxide engages in a chemical reaction with solutions containing ferrous and ferric salts, leading. What Reacts With Sodium Hydroxide.
From www.youtube.com
Phenol and Sodium Hydroxide Reaction C6H5OH + NaOH pH Value Heat of Reaction YouTube What Reacts With Sodium Hydroxide 7 rows are precipitation reactions. In a similar way, sulphuric acid (h 2 so 4). \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +. Naoh + hcl → nacl + h2o. This type of reaction is known as a neutralization reaction. The extraction of excess sodium hydroxide is done with great. So i wanted to know what the reaction between sodium hydroxide and. What Reacts With Sodium Hydroxide.
From www.sciencephoto.com
Sodium hydroxide chemical structure, illustration Stock Image F027/9462 Science Photo Library What Reacts With Sodium Hydroxide For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. The extraction of excess sodium hydroxide is done with great. 7 rows are precipitation reactions. This type of reaction is known as a neutralization reaction. So i wanted to know what the reaction between sodium hydroxide and carbon dioxide can be, and upon research i got. What Reacts With Sodium Hydroxide.
From www.youtube.com
Sodium hydroxide reaction with hydrochloric acid NeutralizationReaction YouTube What Reacts With Sodium Hydroxide The aqueous solution of sodium hydroxide engages in a chemical reaction with solutions containing ferrous and ferric salts, leading to the creation of a visually cloudy. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. In a similar way, sulphuric acid (h 2 so 4). The extraction of excess sodium hydroxide is done with great.. What Reacts With Sodium Hydroxide.
From mungfali.com
Sodium Hydroxide Phase Diagram What Reacts With Sodium Hydroxide Oil and fat triglycerides react with sodium hydroxide to form fatty acid glycerol and sodium salts, the latter being the soaps. \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +. The reaction between sodium hydroxide and hydrochloric acid can be shown in the following equation: Sodium hydroxide is a strong base, meaning it reacts vigorously with acids to form water and a salt.. What Reacts With Sodium Hydroxide.
From www.youtube.com
Write the balanced chemical equation for the reaction of sodium hydroxide with acetic acid 12 What Reacts With Sodium Hydroxide The reaction between sodium hydroxide and hydrochloric acid can be shown in the following equation: \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +. Oil and fat triglycerides react with sodium hydroxide to form fatty acid glycerol and sodium salts, the latter being the soaps. This type of reaction is known as a neutralization reaction. In a similar way, sulphuric acid (h 2. What Reacts With Sodium Hydroxide.
From melscience.com
Sodium hydroxide and reactions with it MEL Chemistry What Reacts With Sodium Hydroxide In a similar way, sulphuric acid (h 2 so 4). 7 rows are precipitation reactions. The reaction between sodium hydroxide and hydrochloric acid can be shown in the following equation: Sodium hydroxide is a strong base, meaning it reacts vigorously with acids to form water and a salt. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and. What Reacts With Sodium Hydroxide.
From www.chegg.com
Solved Reaction 1 The dissolving of solid sodium hydroxide What Reacts With Sodium Hydroxide The extraction of excess sodium hydroxide is done with great. The aqueous solution of sodium hydroxide engages in a chemical reaction with solutions containing ferrous and ferric salts, leading to the creation of a visually cloudy. Naoh + hcl → nacl + h2o. In a similar way, sulphuric acid (h 2 so 4). This type of reaction is known as. What Reacts With Sodium Hydroxide.
From www.youtube.com
Reaction of Sodium Hydroxide and Copper Sulfate YouTube What Reacts With Sodium Hydroxide Naoh + hcl → nacl + h2o. This type of reaction is known as a neutralization reaction. The reaction between sodium hydroxide and hydrochloric acid can be shown in the following equation: Oil and fat triglycerides react with sodium hydroxide to form fatty acid glycerol and sodium salts, the latter being the soaps. The extraction of excess sodium hydroxide is. What Reacts With Sodium Hydroxide.
From www.meritnation.com
Sulphuric acid react with sodium hydroxide as follows when 1L of 0 1M Chemistry Some Basic What Reacts With Sodium Hydroxide The reaction between sodium hydroxide and hydrochloric acid can be shown in the following equation: This type of reaction is known as a neutralization reaction. Oil and fat triglycerides react with sodium hydroxide to form fatty acid glycerol and sodium salts, the latter being the soaps. In a similar way, sulphuric acid (h 2 so 4). 7 rows are precipitation. What Reacts With Sodium Hydroxide.
From www.youtube.com
Equation for NaOH + H2O (Sodium hydroxide + Water) YouTube What Reacts With Sodium Hydroxide So i wanted to know what the reaction between sodium hydroxide and carbon dioxide can be, and upon research i got 2 answers. Sodium hydroxide is a strong base, meaning it reacts vigorously with acids to form water and a salt. This type of reaction is known as a neutralization reaction. 7 rows are precipitation reactions. The aqueous solution of. What Reacts With Sodium Hydroxide.
From www.meritnation.com
What is formed when CuSO4 reacts with Sodium hydroxide solution Also give it's equation What Reacts With Sodium Hydroxide For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas. \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +. Sodium hydroxide is a strong base, meaning it reacts vigorously with acids to form water and a salt. The reaction between sodium hydroxide and hydrochloric acid can be shown in the following equation: Naoh + hcl → nacl + h2o.. What Reacts With Sodium Hydroxide.
From www.youtube.com
Neutralisation Reaction Sulfuric Acid and Sodium Hydroxide Balancing Chemical Reactions H2SO4 What Reacts With Sodium Hydroxide The aqueous solution of sodium hydroxide engages in a chemical reaction with solutions containing ferrous and ferric salts, leading to the creation of a visually cloudy. In a similar way, sulphuric acid (h 2 so 4). The extraction of excess sodium hydroxide is done with great. Oil and fat triglycerides react with sodium hydroxide to form fatty acid glycerol and. What Reacts With Sodium Hydroxide.
From www.chegg.com
Solved Reaction 1 An aqueous solution of sodium hydroxide What Reacts With Sodium Hydroxide Sodium hydroxide is a strong base, meaning it reacts vigorously with acids to form water and a salt. In a similar way, sulphuric acid (h 2 so 4). The extraction of excess sodium hydroxide is done with great. Oil and fat triglycerides react with sodium hydroxide to form fatty acid glycerol and sodium salts, the latter being the soaps. The. What Reacts With Sodium Hydroxide.
From mavink.com
Chemical Formula Of Sodium Hydroxide What Reacts With Sodium Hydroxide In a similar way, sulphuric acid (h 2 so 4). The extraction of excess sodium hydroxide is done with great. 7 rows are precipitation reactions. This type of reaction is known as a neutralization reaction. Sodium hydroxide is a strong base, meaning it reacts vigorously with acids to form water and a salt. \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +. The. What Reacts With Sodium Hydroxide.
From www.numerade.com
SOLVED8.) What is the name of the functional group that sodium hydroxide is reacting with in What Reacts With Sodium Hydroxide The aqueous solution of sodium hydroxide engages in a chemical reaction with solutions containing ferrous and ferric salts, leading to the creation of a visually cloudy. Sodium hydroxide is a strong base, meaning it reacts vigorously with acids to form water and a salt. So i wanted to know what the reaction between sodium hydroxide and carbon dioxide can be,. What Reacts With Sodium Hydroxide.
From brainly.in
What type of salt is obtained when sodium hydroxide reacts with acetic acid? Write with chemical What Reacts With Sodium Hydroxide So i wanted to know what the reaction between sodium hydroxide and carbon dioxide can be, and upon research i got 2 answers. The reaction between sodium hydroxide and hydrochloric acid can be shown in the following equation: \[\ce{zn(s) + 2hcl(aq) → zncl2(aq) +. In a similar way, sulphuric acid (h 2 so 4). 7 rows are precipitation reactions. Naoh. What Reacts With Sodium Hydroxide.
From www.vrogue.co
An Aqueous Solution Of Sodium Hydroxide Reacts Comple vrogue.co What Reacts With Sodium Hydroxide The aqueous solution of sodium hydroxide engages in a chemical reaction with solutions containing ferrous and ferric salts, leading to the creation of a visually cloudy. So i wanted to know what the reaction between sodium hydroxide and carbon dioxide can be, and upon research i got 2 answers. The reaction between sodium hydroxide and hydrochloric acid can be shown. What Reacts With Sodium Hydroxide.