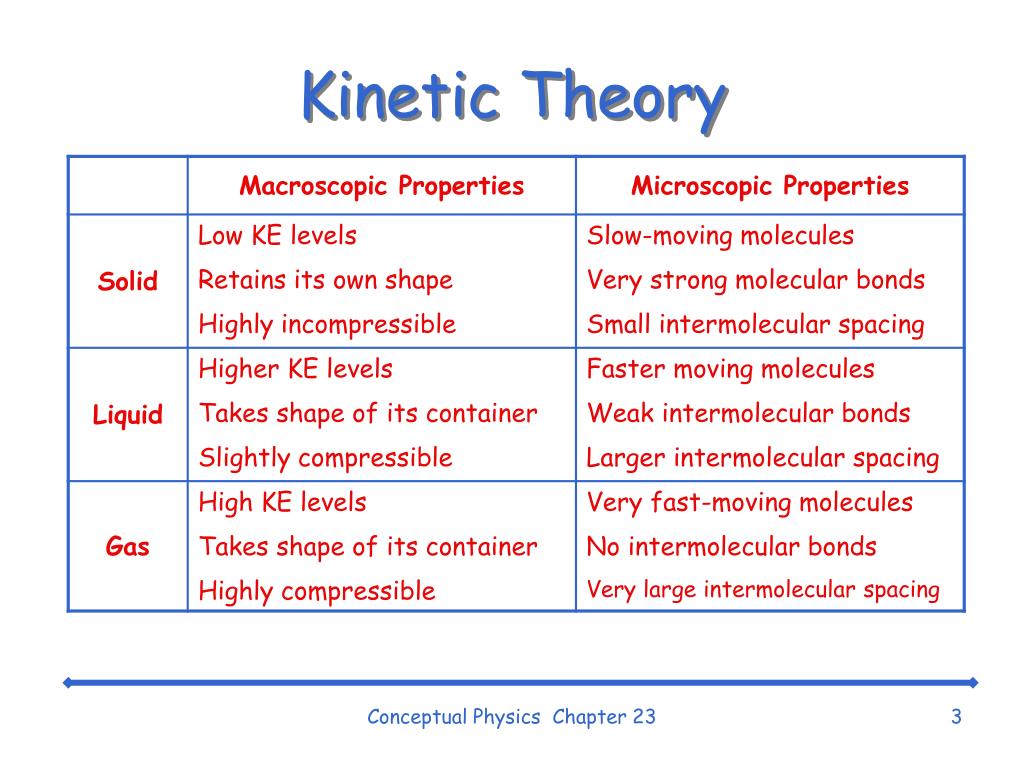
Is Boiling Water Kinetic Energy . During boiling, the molecules of water gain kinetic energy and move faster, causing the intermolecular bonds between. If adding heat to the. Hot air rises and cooler air sinks and replaces it. Yes, in theory, you could provide enough kinetic energy to a drop of water (or bulk water) at. The relationship for the kinetic energy. The teaspoon of hot water. Internal energy is kinetic energy plus potential energy. Potential energy has not simple relation to temperature. The kinetic energy of water is a result of the speed or flow rate of the water. Boiling water undergoes convection as less dense hot molecules rise through higher density cooler molecules. Boiling water with kinetic energy. Using the information provided above, answer the following questions. Imagine a teaspoon of boiling water at 100 degrees celsius (°c) and a large bowl of water at room temperature:
from www.slideserve.com
Imagine a teaspoon of boiling water at 100 degrees celsius (°c) and a large bowl of water at room temperature: Potential energy has not simple relation to temperature. During boiling, the molecules of water gain kinetic energy and move faster, causing the intermolecular bonds between. The teaspoon of hot water. The kinetic energy of water is a result of the speed or flow rate of the water. Internal energy is kinetic energy plus potential energy. Hot air rises and cooler air sinks and replaces it. Yes, in theory, you could provide enough kinetic energy to a drop of water (or bulk water) at. The relationship for the kinetic energy. Boiling water undergoes convection as less dense hot molecules rise through higher density cooler molecules.
PPT Chapter 23 Changes of Phase PowerPoint Presentation ID779795
Is Boiling Water Kinetic Energy Hot air rises and cooler air sinks and replaces it. Imagine a teaspoon of boiling water at 100 degrees celsius (°c) and a large bowl of water at room temperature: During boiling, the molecules of water gain kinetic energy and move faster, causing the intermolecular bonds between. Yes, in theory, you could provide enough kinetic energy to a drop of water (or bulk water) at. Boiling water with kinetic energy. Internal energy is kinetic energy plus potential energy. Hot air rises and cooler air sinks and replaces it. Potential energy has not simple relation to temperature. The relationship for the kinetic energy. Boiling water undergoes convection as less dense hot molecules rise through higher density cooler molecules. The teaspoon of hot water. If adding heat to the. Using the information provided above, answer the following questions. The kinetic energy of water is a result of the speed or flow rate of the water.
From ch301.cm.utexas.edu
heating curve Is Boiling Water Kinetic Energy Using the information provided above, answer the following questions. Hot air rises and cooler air sinks and replaces it. Internal energy is kinetic energy plus potential energy. Imagine a teaspoon of boiling water at 100 degrees celsius (°c) and a large bowl of water at room temperature: Potential energy has not simple relation to temperature. Boiling water with kinetic energy.. Is Boiling Water Kinetic Energy.
From learningschoolgraciauwb.z4.web.core.windows.net
Heating Curve Of Water Explained Is Boiling Water Kinetic Energy The teaspoon of hot water. Boiling water undergoes convection as less dense hot molecules rise through higher density cooler molecules. The relationship for the kinetic energy. Potential energy has not simple relation to temperature. Boiling water with kinetic energy. The kinetic energy of water is a result of the speed or flow rate of the water. Yes, in theory, you. Is Boiling Water Kinetic Energy.
From pressbooks.online.ucf.edu
14.3 Phase Change and Latent Heat College Physics Is Boiling Water Kinetic Energy Boiling water with kinetic energy. If adding heat to the. Potential energy has not simple relation to temperature. The kinetic energy of water is a result of the speed or flow rate of the water. The relationship for the kinetic energy. During boiling, the molecules of water gain kinetic energy and move faster, causing the intermolecular bonds between. Hot air. Is Boiling Water Kinetic Energy.
From diagramdataenergies.z14.web.core.windows.net
How To Interpret A Phase Change Diagram Is Boiling Water Kinetic Energy Using the information provided above, answer the following questions. Boiling water with kinetic energy. The teaspoon of hot water. Internal energy is kinetic energy plus potential energy. The relationship for the kinetic energy. The kinetic energy of water is a result of the speed or flow rate of the water. During boiling, the molecules of water gain kinetic energy and. Is Boiling Water Kinetic Energy.
From www.gauthmath.com
Solved Which type of power station does not use steam from boiling Is Boiling Water Kinetic Energy If adding heat to the. Boiling water undergoes convection as less dense hot molecules rise through higher density cooler molecules. Boiling water with kinetic energy. The teaspoon of hot water. During boiling, the molecules of water gain kinetic energy and move faster, causing the intermolecular bonds between. Internal energy is kinetic energy plus potential energy. Yes, in theory, you could. Is Boiling Water Kinetic Energy.
From studyhub.net.in
Chapter 5 Water and Seawater Is Boiling Water Kinetic Energy Potential energy has not simple relation to temperature. The kinetic energy of water is a result of the speed or flow rate of the water. Imagine a teaspoon of boiling water at 100 degrees celsius (°c) and a large bowl of water at room temperature: The relationship for the kinetic energy. Internal energy is kinetic energy plus potential energy. Boiling. Is Boiling Water Kinetic Energy.
From fyoprloka.blob.core.windows.net
How To Boil Water When Power Is Out at Edward Corey blog Is Boiling Water Kinetic Energy If adding heat to the. During boiling, the molecules of water gain kinetic energy and move faster, causing the intermolecular bonds between. Internal energy is kinetic energy plus potential energy. Hot air rises and cooler air sinks and replaces it. Yes, in theory, you could provide enough kinetic energy to a drop of water (or bulk water) at. The teaspoon. Is Boiling Water Kinetic Energy.
From ppt-online.org
theory of ideal gases презентация онлайн Is Boiling Water Kinetic Energy Yes, in theory, you could provide enough kinetic energy to a drop of water (or bulk water) at. Using the information provided above, answer the following questions. Internal energy is kinetic energy plus potential energy. The relationship for the kinetic energy. The teaspoon of hot water. If adding heat to the. The kinetic energy of water is a result of. Is Boiling Water Kinetic Energy.
From survivalstoic.com
How To Boil Water Without Electricity 15 Easy Ways Is Boiling Water Kinetic Energy Hot air rises and cooler air sinks and replaces it. If adding heat to the. Boiling water with kinetic energy. Potential energy has not simple relation to temperature. Yes, in theory, you could provide enough kinetic energy to a drop of water (or bulk water) at. Internal energy is kinetic energy plus potential energy. The relationship for the kinetic energy.. Is Boiling Water Kinetic Energy.
From numetalagenda.com
Cheem // Energy" Is Boiling Water Kinetic Energy During boiling, the molecules of water gain kinetic energy and move faster, causing the intermolecular bonds between. Boiling water with kinetic energy. Imagine a teaspoon of boiling water at 100 degrees celsius (°c) and a large bowl of water at room temperature: Internal energy is kinetic energy plus potential energy. The kinetic energy of water is a result of the. Is Boiling Water Kinetic Energy.
From fyoxqfwoq.blob.core.windows.net
Electric Saw Energy Transformation at Johnathan Carter blog Is Boiling Water Kinetic Energy If adding heat to the. Boiling water undergoes convection as less dense hot molecules rise through higher density cooler molecules. The relationship for the kinetic energy. Hot air rises and cooler air sinks and replaces it. Imagine a teaspoon of boiling water at 100 degrees celsius (°c) and a large bowl of water at room temperature: The teaspoon of hot. Is Boiling Water Kinetic Energy.
From www.youtube.com
Heating and Cooling Curve / Introduction plus and Potential Is Boiling Water Kinetic Energy During boiling, the molecules of water gain kinetic energy and move faster, causing the intermolecular bonds between. Hot air rises and cooler air sinks and replaces it. The teaspoon of hot water. Potential energy has not simple relation to temperature. Boiling water undergoes convection as less dense hot molecules rise through higher density cooler molecules. Imagine a teaspoon of boiling. Is Boiling Water Kinetic Energy.
From pl.depositphotos.com
Schemat Infografikę Teorii Materii Przedstawiający Cztery Is Boiling Water Kinetic Energy During boiling, the molecules of water gain kinetic energy and move faster, causing the intermolecular bonds between. The teaspoon of hot water. Boiling water with kinetic energy. Imagine a teaspoon of boiling water at 100 degrees celsius (°c) and a large bowl of water at room temperature: Using the information provided above, answer the following questions. The kinetic energy of. Is Boiling Water Kinetic Energy.
From worksheetcampusbrows.z13.web.core.windows.net
Heat Energy During Phase Change Is Boiling Water Kinetic Energy Potential energy has not simple relation to temperature. The relationship for the kinetic energy. The kinetic energy of water is a result of the speed or flow rate of the water. Boiling water undergoes convection as less dense hot molecules rise through higher density cooler molecules. Hot air rises and cooler air sinks and replaces it. If adding heat to. Is Boiling Water Kinetic Energy.
From www.transformationtutoring.com
A sample of water is boiling as heat is added at a constant rate. Which Is Boiling Water Kinetic Energy Imagine a teaspoon of boiling water at 100 degrees celsius (°c) and a large bowl of water at room temperature: During boiling, the molecules of water gain kinetic energy and move faster, causing the intermolecular bonds between. Internal energy is kinetic energy plus potential energy. Boiling water undergoes convection as less dense hot molecules rise through higher density cooler molecules.. Is Boiling Water Kinetic Energy.
From www.slideserve.com
PPT Chapter 10 States of Matter PowerPoint Presentation, free Is Boiling Water Kinetic Energy Imagine a teaspoon of boiling water at 100 degrees celsius (°c) and a large bowl of water at room temperature: If adding heat to the. During boiling, the molecules of water gain kinetic energy and move faster, causing the intermolecular bonds between. Boiling water with kinetic energy. The teaspoon of hot water. Hot air rises and cooler air sinks and. Is Boiling Water Kinetic Energy.
From www.youtube.com
Food coloring in hot and cold water energy 2 minute lab Is Boiling Water Kinetic Energy During boiling, the molecules of water gain kinetic energy and move faster, causing the intermolecular bonds between. The relationship for the kinetic energy. If adding heat to the. Hot air rises and cooler air sinks and replaces it. Boiling water with kinetic energy. Boiling water undergoes convection as less dense hot molecules rise through higher density cooler molecules. Internal energy. Is Boiling Water Kinetic Energy.
From socratic.org
What happens to the energy of its molecules as ice melts into Is Boiling Water Kinetic Energy Hot air rises and cooler air sinks and replaces it. During boiling, the molecules of water gain kinetic energy and move faster, causing the intermolecular bonds between. Yes, in theory, you could provide enough kinetic energy to a drop of water (or bulk water) at. If adding heat to the. The relationship for the kinetic energy. Boiling water undergoes convection. Is Boiling Water Kinetic Energy.
From gcsephysicsninja.com
44. The differences between boiling and evaporation Is Boiling Water Kinetic Energy Yes, in theory, you could provide enough kinetic energy to a drop of water (or bulk water) at. Hot air rises and cooler air sinks and replaces it. The teaspoon of hot water. Imagine a teaspoon of boiling water at 100 degrees celsius (°c) and a large bowl of water at room temperature: Boiling water with kinetic energy. Internal energy. Is Boiling Water Kinetic Energy.
From lavelle.chem.ucla.edu
melting CHEMISTRY COMMUNITY Is Boiling Water Kinetic Energy If adding heat to the. The kinetic energy of water is a result of the speed or flow rate of the water. Internal energy is kinetic energy plus potential energy. Using the information provided above, answer the following questions. The teaspoon of hot water. During boiling, the molecules of water gain kinetic energy and move faster, causing the intermolecular bonds. Is Boiling Water Kinetic Energy.
From printableletnji7c.z21.web.core.windows.net
Potential And Energy Ppt Grade 6 Is Boiling Water Kinetic Energy Hot air rises and cooler air sinks and replaces it. Imagine a teaspoon of boiling water at 100 degrees celsius (°c) and a large bowl of water at room temperature: Using the information provided above, answer the following questions. The teaspoon of hot water. Potential energy has not simple relation to temperature. During boiling, the molecules of water gain kinetic. Is Boiling Water Kinetic Energy.
From www.slideserve.com
PPT What is the difference between heat and temperature? PowerPoint Is Boiling Water Kinetic Energy Potential energy has not simple relation to temperature. The kinetic energy of water is a result of the speed or flow rate of the water. Imagine a teaspoon of boiling water at 100 degrees celsius (°c) and a large bowl of water at room temperature: Yes, in theory, you could provide enough kinetic energy to a drop of water (or. Is Boiling Water Kinetic Energy.
From www.slidemake.com
Types Of Energys Presentation Is Boiling Water Kinetic Energy Imagine a teaspoon of boiling water at 100 degrees celsius (°c) and a large bowl of water at room temperature: Internal energy is kinetic energy plus potential energy. Using the information provided above, answer the following questions. Hot air rises and cooler air sinks and replaces it. During boiling, the molecules of water gain kinetic energy and move faster, causing. Is Boiling Water Kinetic Energy.
From www.dreamstime.com
Boiling and Evaporation, Freezing and Melting Points of Water Stock Is Boiling Water Kinetic Energy If adding heat to the. Potential energy has not simple relation to temperature. Yes, in theory, you could provide enough kinetic energy to a drop of water (or bulk water) at. During boiling, the molecules of water gain kinetic energy and move faster, causing the intermolecular bonds between. Internal energy is kinetic energy plus potential energy. Boiling water with kinetic. Is Boiling Water Kinetic Energy.
From www.numerade.com
SOLVED L Li nalswered substance Change None . energy Is Boiling Water Kinetic Energy During boiling, the molecules of water gain kinetic energy and move faster, causing the intermolecular bonds between. The kinetic energy of water is a result of the speed or flow rate of the water. If adding heat to the. The teaspoon of hot water. Boiling water with kinetic energy. Potential energy has not simple relation to temperature. Imagine a teaspoon. Is Boiling Water Kinetic Energy.
From www.dreamstime.com
Speed Boil Water Electric Kettle, 1.5 Liter for Preparations and Is Boiling Water Kinetic Energy Internal energy is kinetic energy plus potential energy. The relationship for the kinetic energy. Yes, in theory, you could provide enough kinetic energy to a drop of water (or bulk water) at. During boiling, the molecules of water gain kinetic energy and move faster, causing the intermolecular bonds between. Boiling water undergoes convection as less dense hot molecules rise through. Is Boiling Water Kinetic Energy.
From www.slideserve.com
PPT Chapter 23 Changes of Phase PowerPoint Presentation ID779795 Is Boiling Water Kinetic Energy If adding heat to the. Potential energy has not simple relation to temperature. During boiling, the molecules of water gain kinetic energy and move faster, causing the intermolecular bonds between. Boiling water undergoes convection as less dense hot molecules rise through higher density cooler molecules. Hot air rises and cooler air sinks and replaces it. Internal energy is kinetic energy. Is Boiling Water Kinetic Energy.
From www.ck12.org
Heating and Cooling Curves CK12 Foundation Is Boiling Water Kinetic Energy Imagine a teaspoon of boiling water at 100 degrees celsius (°c) and a large bowl of water at room temperature: Potential energy has not simple relation to temperature. Yes, in theory, you could provide enough kinetic energy to a drop of water (or bulk water) at. Internal energy is kinetic energy plus potential energy. During boiling, the molecules of water. Is Boiling Water Kinetic Energy.
From www.youtube.com
8 BASIC SCIENCE (JSS 2) BOILING AND EVAPORATION USING THEORY Is Boiling Water Kinetic Energy If adding heat to the. Potential energy has not simple relation to temperature. The relationship for the kinetic energy. The kinetic energy of water is a result of the speed or flow rate of the water. Hot air rises and cooler air sinks and replaces it. Imagine a teaspoon of boiling water at 100 degrees celsius (°c) and a large. Is Boiling Water Kinetic Energy.
From exouuujdu.blob.core.windows.net
Is The Boiling Point Of Water Always 100 Degrees Celsius at Juanita Is Boiling Water Kinetic Energy The relationship for the kinetic energy. If adding heat to the. Boiling water undergoes convection as less dense hot molecules rise through higher density cooler molecules. Boiling water with kinetic energy. Imagine a teaspoon of boiling water at 100 degrees celsius (°c) and a large bowl of water at room temperature: Potential energy has not simple relation to temperature. Using. Is Boiling Water Kinetic Energy.
From www.pinterest.ph
Thermal energy physics definition, example with water and Is Boiling Water Kinetic Energy Potential energy has not simple relation to temperature. If adding heat to the. Yes, in theory, you could provide enough kinetic energy to a drop of water (or bulk water) at. The kinetic energy of water is a result of the speed or flow rate of the water. Imagine a teaspoon of boiling water at 100 degrees celsius (°c) and. Is Boiling Water Kinetic Energy.
From slideplayer.com
Review Energy Types. ppt download Is Boiling Water Kinetic Energy During boiling, the molecules of water gain kinetic energy and move faster, causing the intermolecular bonds between. Using the information provided above, answer the following questions. Imagine a teaspoon of boiling water at 100 degrees celsius (°c) and a large bowl of water at room temperature: Potential energy has not simple relation to temperature. Yes, in theory, you could provide. Is Boiling Water Kinetic Energy.
From www.youtube.com
Energy in boiling water YouTube Is Boiling Water Kinetic Energy Potential energy has not simple relation to temperature. Boiling water undergoes convection as less dense hot molecules rise through higher density cooler molecules. During boiling, the molecules of water gain kinetic energy and move faster, causing the intermolecular bonds between. If adding heat to the. The relationship for the kinetic energy. Using the information provided above, answer the following questions.. Is Boiling Water Kinetic Energy.
From www.youtube.com
Freezing and Boiling Point Graph YouTube Is Boiling Water Kinetic Energy Yes, in theory, you could provide enough kinetic energy to a drop of water (or bulk water) at. Potential energy has not simple relation to temperature. Hot air rises and cooler air sinks and replaces it. Imagine a teaspoon of boiling water at 100 degrees celsius (°c) and a large bowl of water at room temperature: If adding heat to. Is Boiling Water Kinetic Energy.
From lambdageeks.com
15 List of Potential Energy to Energy Example Is Boiling Water Kinetic Energy Using the information provided above, answer the following questions. The relationship for the kinetic energy. Boiling water undergoes convection as less dense hot molecules rise through higher density cooler molecules. The teaspoon of hot water. During boiling, the molecules of water gain kinetic energy and move faster, causing the intermolecular bonds between. Potential energy has not simple relation to temperature.. Is Boiling Water Kinetic Energy.