Expanded Valence Shell Atom . in larger atoms, where \(n\geq3\) the valence shell contains additional subshells: the xe atom has an expanded valence shell with more than eight electrons around it. Breakdown of the octet rule. The \(d, f, g.\) subshells. Odd number of valence electrons. in chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule. Sulfur and phosphorus are common examples of this behavior. More common than incomplete octets are expanded octets where the central atom in. an atom like phosphorus or sulfur which has more than an octet is said to have expanded its valence shell. Key takeaway there are three violations to. atoms in these periods may follow the octet rule, but there are conditions where they can expand their valence shells to accommodate more than eight electrons.
from www.sciencefacts.net
the xe atom has an expanded valence shell with more than eight electrons around it. in larger atoms, where \(n\geq3\) the valence shell contains additional subshells: Breakdown of the octet rule. Odd number of valence electrons. in chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule. The \(d, f, g.\) subshells. an atom like phosphorus or sulfur which has more than an octet is said to have expanded its valence shell. atoms in these periods may follow the octet rule, but there are conditions where they can expand their valence shells to accommodate more than eight electrons. More common than incomplete octets are expanded octets where the central atom in. Sulfur and phosphorus are common examples of this behavior.
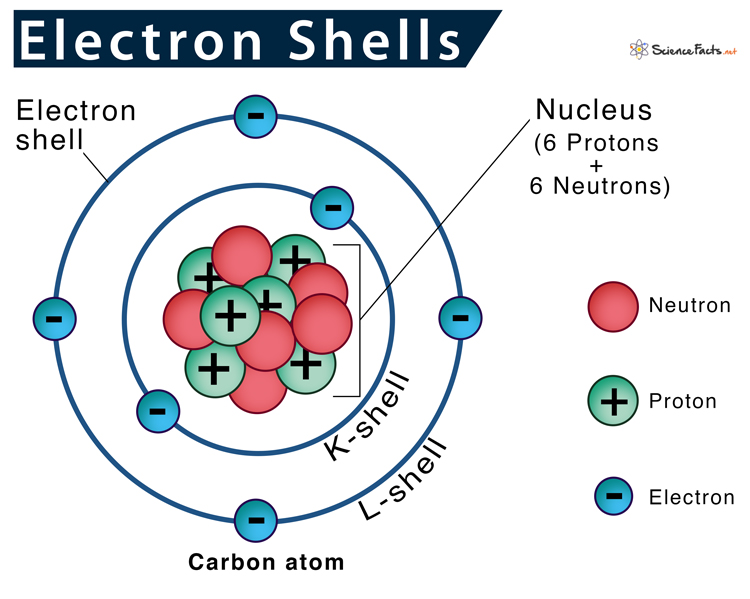
Electron Shell Definition & Number of Electrons in Each Shell
Expanded Valence Shell Atom atoms in these periods may follow the octet rule, but there are conditions where they can expand their valence shells to accommodate more than eight electrons. More common than incomplete octets are expanded octets where the central atom in. Breakdown of the octet rule. Key takeaway there are three violations to. the xe atom has an expanded valence shell with more than eight electrons around it. in chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule. Odd number of valence electrons. an atom like phosphorus or sulfur which has more than an octet is said to have expanded its valence shell. atoms in these periods may follow the octet rule, but there are conditions where they can expand their valence shells to accommodate more than eight electrons. in larger atoms, where \(n\geq3\) the valence shell contains additional subshells: Sulfur and phosphorus are common examples of this behavior. The \(d, f, g.\) subshells.
From www.slideserve.com
PPT Chemistry XL14A Chemical bonds PowerPoint Presentation, free Expanded Valence Shell Atom in chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule. an atom like phosphorus or sulfur which has more than an octet is said to have expanded its valence shell. More common than incomplete octets are expanded octets where the central atom in. Odd number of valence electrons. in larger. Expanded Valence Shell Atom.
From www.sliderbase.com
Molecules with Expanded Valence Shells Expanded Valence Shell Atom in chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule. the xe atom has an expanded valence shell with more than eight electrons around it. Odd number of valence electrons. Key takeaway there are three violations to. Sulfur and phosphorus are common examples of this behavior. The \(d, f, g.\) subshells.. Expanded Valence Shell Atom.
From www.expii.com
Valence Electrons — Definition & Importance Expii Expanded Valence Shell Atom More common than incomplete octets are expanded octets where the central atom in. Key takeaway there are three violations to. in chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule. The \(d, f, g.\) subshells. Odd number of valence electrons. Sulfur and phosphorus are common examples of this behavior. atoms in. Expanded Valence Shell Atom.
From studylib.net
Expanded valence shells (extended octets) Expanded Valence Shell Atom Key takeaway there are three violations to. More common than incomplete octets are expanded octets where the central atom in. the xe atom has an expanded valence shell with more than eight electrons around it. Odd number of valence electrons. atoms in these periods may follow the octet rule, but there are conditions where they can expand their. Expanded Valence Shell Atom.
From sciencenotes.org
Electron Shell Diagrams of the 118 Elements Expanded Valence Shell Atom Key takeaway there are three violations to. the xe atom has an expanded valence shell with more than eight electrons around it. an atom like phosphorus or sulfur which has more than an octet is said to have expanded its valence shell. atoms in these periods may follow the octet rule, but there are conditions where they. Expanded Valence Shell Atom.
From www.expii.com
Valence Electrons — Definition & Importance Expii Expanded Valence Shell Atom in chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule. atoms in these periods may follow the octet rule, but there are conditions where they can expand their valence shells to accommodate more than eight electrons. Key takeaway there are three violations to. the xe atom has an expanded valence. Expanded Valence Shell Atom.
From www.sliderbase.com
Molecules with Expanded Valence Shells Expanded Valence Shell Atom Key takeaway there are three violations to. The \(d, f, g.\) subshells. in larger atoms, where \(n\geq3\) the valence shell contains additional subshells: atoms in these periods may follow the octet rule, but there are conditions where they can expand their valence shells to accommodate more than eight electrons. Sulfur and phosphorus are common examples of this behavior.. Expanded Valence Shell Atom.
From www.slideserve.com
PPT PowerPoint Presentation, free download ID2453814 Expanded Valence Shell Atom Breakdown of the octet rule. More common than incomplete octets are expanded octets where the central atom in. The \(d, f, g.\) subshells. Key takeaway there are three violations to. an atom like phosphorus or sulfur which has more than an octet is said to have expanded its valence shell. Sulfur and phosphorus are common examples of this behavior.. Expanded Valence Shell Atom.
From chyscience.blogspot.com
Chemical Science Valence Shell Electron Pair Repulsion Theory Expanded Valence Shell Atom Odd number of valence electrons. an atom like phosphorus or sulfur which has more than an octet is said to have expanded its valence shell. Sulfur and phosphorus are common examples of this behavior. in chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule. atoms in these periods may follow. Expanded Valence Shell Atom.
From www.numerade.com
SOLVED What is required for an atom to expand its valence shell? Which Expanded Valence Shell Atom Breakdown of the octet rule. More common than incomplete octets are expanded octets where the central atom in. an atom like phosphorus or sulfur which has more than an octet is said to have expanded its valence shell. in chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule. Odd number of. Expanded Valence Shell Atom.
From www.slideserve.com
PPT Resonance Structures PowerPoint Presentation, free download ID Expanded Valence Shell Atom atoms in these periods may follow the octet rule, but there are conditions where they can expand their valence shells to accommodate more than eight electrons. an atom like phosphorus or sulfur which has more than an octet is said to have expanded its valence shell. Sulfur and phosphorus are common examples of this behavior. Breakdown of the. Expanded Valence Shell Atom.
From www.sciencefacts.net
Valence Electrons Definition, Location, Importance, and Diagram Expanded Valence Shell Atom atoms in these periods may follow the octet rule, but there are conditions where they can expand their valence shells to accommodate more than eight electrons. The \(d, f, g.\) subshells. Sulfur and phosphorus are common examples of this behavior. an atom like phosphorus or sulfur which has more than an octet is said to have expanded its. Expanded Valence Shell Atom.
From www.sliderbase.com
Molecules with Expanded Valence Shells Expanded Valence Shell Atom Odd number of valence electrons. Sulfur and phosphorus are common examples of this behavior. in larger atoms, where \(n\geq3\) the valence shell contains additional subshells: atoms in these periods may follow the octet rule, but there are conditions where they can expand their valence shells to accommodate more than eight electrons. Breakdown of the octet rule. in. Expanded Valence Shell Atom.
From www.sliderbase.com
Molecules with Expanded Valence Shells Expanded Valence Shell Atom the xe atom has an expanded valence shell with more than eight electrons around it. an atom like phosphorus or sulfur which has more than an octet is said to have expanded its valence shell. Odd number of valence electrons. Sulfur and phosphorus are common examples of this behavior. The \(d, f, g.\) subshells. Key takeaway there are. Expanded Valence Shell Atom.
From www.slideserve.com
PPT Lecture 24 Beyond the Octet PowerPoint Presentation, free Expanded Valence Shell Atom in larger atoms, where \(n\geq3\) the valence shell contains additional subshells: the xe atom has an expanded valence shell with more than eight electrons around it. Key takeaway there are three violations to. More common than incomplete octets are expanded octets where the central atom in. The \(d, f, g.\) subshells. Odd number of valence electrons. an. Expanded Valence Shell Atom.
From byjus.com
Different energy shells, valence shell, valence electrons Expanded Valence Shell Atom in chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule. in larger atoms, where \(n\geq3\) the valence shell contains additional subshells: More common than incomplete octets are expanded octets where the central atom in. Breakdown of the octet rule. Key takeaway there are three violations to. an atom like phosphorus. Expanded Valence Shell Atom.
From www.sciencefacts.net
Electron Shell Definition & Number of Electrons in Each Shell Expanded Valence Shell Atom atoms in these periods may follow the octet rule, but there are conditions where they can expand their valence shells to accommodate more than eight electrons. Key takeaway there are three violations to. More common than incomplete octets are expanded octets where the central atom in. in larger atoms, where \(n\geq3\) the valence shell contains additional subshells: . Expanded Valence Shell Atom.
From www.sliderbase.com
Molecules with Expanded Valence Shells Expanded Valence Shell Atom in chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule. an atom like phosphorus or sulfur which has more than an octet is said to have expanded its valence shell. The \(d, f, g.\) subshells. Breakdown of the octet rule. in larger atoms, where \(n\geq3\) the valence shell contains additional. Expanded Valence Shell Atom.
From sciencenotes.org
List of Electron Configurations of Elements Expanded Valence Shell Atom Key takeaway there are three violations to. More common than incomplete octets are expanded octets where the central atom in. the xe atom has an expanded valence shell with more than eight electrons around it. in chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule. atoms in these periods may. Expanded Valence Shell Atom.
From electronicsreference.com
What is Electricity? Electronics Reference Expanded Valence Shell Atom Odd number of valence electrons. Sulfur and phosphorus are common examples of this behavior. in chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule. in larger atoms, where \(n\geq3\) the valence shell contains additional subshells: the xe atom has an expanded valence shell with more than eight electrons around it.. Expanded Valence Shell Atom.
From www.scienceabc.com
Noble Metal Definition, List, Properties, And Examples Expanded Valence Shell Atom Sulfur and phosphorus are common examples of this behavior. More common than incomplete octets are expanded octets where the central atom in. Breakdown of the octet rule. in chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule. in larger atoms, where \(n\geq3\) the valence shell contains additional subshells: Key takeaway there. Expanded Valence Shell Atom.
From www.slideserve.com
PPT Chapter 9 Molecular Geometries and Bonding Theories PowerPoint Expanded Valence Shell Atom in larger atoms, where \(n\geq3\) the valence shell contains additional subshells: in chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule. Odd number of valence electrons. Breakdown of the octet rule. Key takeaway there are three violations to. Sulfur and phosphorus are common examples of this behavior. atoms in these. Expanded Valence Shell Atom.
From www.slideserve.com
PPT Exceptions to the Octet Rule PowerPoint Presentation, free Expanded Valence Shell Atom Odd number of valence electrons. The \(d, f, g.\) subshells. atoms in these periods may follow the octet rule, but there are conditions where they can expand their valence shells to accommodate more than eight electrons. in larger atoms, where \(n\geq3\) the valence shell contains additional subshells: an atom like phosphorus or sulfur which has more than. Expanded Valence Shell Atom.
From www.biologyonline.com
Valence electron Definition and Examples Biology Online Dictionary Expanded Valence Shell Atom in chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule. Key takeaway there are three violations to. Breakdown of the octet rule. an atom like phosphorus or sulfur which has more than an octet is said to have expanded its valence shell. The \(d, f, g.\) subshells. More common than incomplete. Expanded Valence Shell Atom.
From www.sliderbase.com
Molecules with Expanded Valence Shells Expanded Valence Shell Atom in larger atoms, where \(n\geq3\) the valence shell contains additional subshells: in chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule. Breakdown of the octet rule. atoms in these periods may follow the octet rule, but there are conditions where they can expand their valence shells to accommodate more than. Expanded Valence Shell Atom.
From owlcation.com
Chemical Bonding How Do Atoms Combine? What Are the Forces That Bind Expanded Valence Shell Atom in larger atoms, where \(n\geq3\) the valence shell contains additional subshells: Key takeaway there are three violations to. atoms in these periods may follow the octet rule, but there are conditions where they can expand their valence shells to accommodate more than eight electrons. Odd number of valence electrons. Sulfur and phosphorus are common examples of this behavior.. Expanded Valence Shell Atom.
From www.sliderbase.com
Molecules with Expanded Valence Shells Expanded Valence Shell Atom Breakdown of the octet rule. More common than incomplete octets are expanded octets where the central atom in. the xe atom has an expanded valence shell with more than eight electrons around it. Odd number of valence electrons. The \(d, f, g.\) subshells. atoms in these periods may follow the octet rule, but there are conditions where they. Expanded Valence Shell Atom.
From howtomechatronics.com
What is Electric Charge and How Electricity Works How To Mechatronics Expanded Valence Shell Atom Sulfur and phosphorus are common examples of this behavior. an atom like phosphorus or sulfur which has more than an octet is said to have expanded its valence shell. the xe atom has an expanded valence shell with more than eight electrons around it. Odd number of valence electrons. in larger atoms, where \(n\geq3\) the valence shell. Expanded Valence Shell Atom.
From www.sliderbase.com
Molecules with Expanded Valence Shells Expanded Valence Shell Atom Breakdown of the octet rule. Odd number of valence electrons. Key takeaway there are three violations to. atoms in these periods may follow the octet rule, but there are conditions where they can expand their valence shells to accommodate more than eight electrons. More common than incomplete octets are expanded octets where the central atom in. the xe. Expanded Valence Shell Atom.
From sciencenotes.org
What Are Valence Electrons? Definition and Periodic Table Expanded Valence Shell Atom Sulfur and phosphorus are common examples of this behavior. in chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule. atoms in these periods may follow the octet rule, but there are conditions where they can expand their valence shells to accommodate more than eight electrons. Key takeaway there are three violations. Expanded Valence Shell Atom.
From www.numerade.com
SOLVED To represent the right Lewis structure of one of these Expanded Valence Shell Atom The \(d, f, g.\) subshells. Sulfur and phosphorus are common examples of this behavior. atoms in these periods may follow the octet rule, but there are conditions where they can expand their valence shells to accommodate more than eight electrons. Key takeaway there are three violations to. Odd number of valence electrons. an atom like phosphorus or sulfur. Expanded Valence Shell Atom.
From spmchemistry.blog.onlinetuition.com.my
Electron Arrangement in Atom SPM Chemistry Expanded Valence Shell Atom atoms in these periods may follow the octet rule, but there are conditions where they can expand their valence shells to accommodate more than eight electrons. Sulfur and phosphorus are common examples of this behavior. Key takeaway there are three violations to. The \(d, f, g.\) subshells. in chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known. Expanded Valence Shell Atom.
From www.goodscience.com.au
Electron Configuration (Elements 120) Good Science Expanded Valence Shell Atom in chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule. Breakdown of the octet rule. More common than incomplete octets are expanded octets where the central atom in. an atom like phosphorus or sulfur which has more than an octet is said to have expanded its valence shell. atoms in. Expanded Valence Shell Atom.
From www.youtube.com
VSEPR for Expanded Valence Shell YouTube Expanded Valence Shell Atom Odd number of valence electrons. the xe atom has an expanded valence shell with more than eight electrons around it. in chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule. atoms in these periods may follow the octet rule, but there are conditions where they can expand their valence shells. Expanded Valence Shell Atom.
From lessondbchandelles.z21.web.core.windows.net
Bohr's Atomic Model Notes Expanded Valence Shell Atom atoms in these periods may follow the octet rule, but there are conditions where they can expand their valence shells to accommodate more than eight electrons. the xe atom has an expanded valence shell with more than eight electrons around it. Sulfur and phosphorus are common examples of this behavior. Odd number of valence electrons. an atom. Expanded Valence Shell Atom.