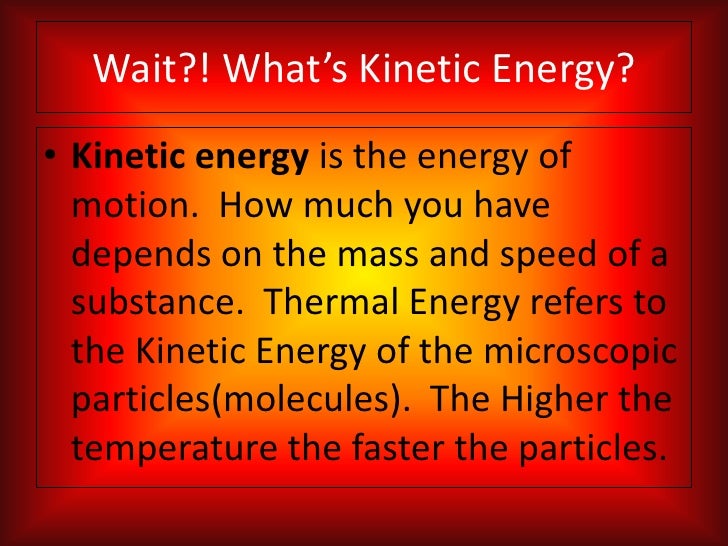
How Is Thermal Energy Kinetic . The thermal energy is the. For example a ball falling has kinetic energy. kinetic energy is the total energy of a particle or system. the idea of thermal energy is derived from the kinetic molecular theory of matter (kmtom). the system starts with kinetic energy, which goes away, replaced with thermal energy. Particles can have translational, rotational,. thermal energy, internal energy present in a system in a state of thermodynamic equilibrium by virtue of its temperature. the thermal energy is the total sum of the potential energies of the particles in a system. Thermal energy cannot be converted to useful work as easily as the energy of systems that are not in states of thermodynamic equilibrium. This happens by means of work done by kinetic friction, so this energy transfer can be treated in the same way as if it were transferred as heat. the kinetic temperature is the variable needed for subjects like heat transfer, because it is the translational kinetic. thermal energy refers to the kinetic energy of randomly moving particles in a substance.
from www.slideshare.net
the thermal energy is the total sum of the potential energies of the particles in a system. thermal energy refers to the kinetic energy of randomly moving particles in a substance. Particles can have translational, rotational,. the kinetic temperature is the variable needed for subjects like heat transfer, because it is the translational kinetic. This happens by means of work done by kinetic friction, so this energy transfer can be treated in the same way as if it were transferred as heat. the system starts with kinetic energy, which goes away, replaced with thermal energy. thermal energy, internal energy present in a system in a state of thermodynamic equilibrium by virtue of its temperature. For example a ball falling has kinetic energy. kinetic energy is the total energy of a particle or system. the idea of thermal energy is derived from the kinetic molecular theory of matter (kmtom).
Thermal Energy And You
How Is Thermal Energy Kinetic kinetic energy is the total energy of a particle or system. The thermal energy is the. the idea of thermal energy is derived from the kinetic molecular theory of matter (kmtom). Particles can have translational, rotational,. kinetic energy is the total energy of a particle or system. thermal energy, internal energy present in a system in a state of thermodynamic equilibrium by virtue of its temperature. For example a ball falling has kinetic energy. Thermal energy cannot be converted to useful work as easily as the energy of systems that are not in states of thermodynamic equilibrium. This happens by means of work done by kinetic friction, so this energy transfer can be treated in the same way as if it were transferred as heat. the system starts with kinetic energy, which goes away, replaced with thermal energy. thermal energy refers to the kinetic energy of randomly moving particles in a substance. the kinetic temperature is the variable needed for subjects like heat transfer, because it is the translational kinetic. the thermal energy is the total sum of the potential energies of the particles in a system.
From www.physicsfox.org
Energy Forms • Energy • Physics Fox How Is Thermal Energy Kinetic thermal energy refers to the kinetic energy of randomly moving particles in a substance. the idea of thermal energy is derived from the kinetic molecular theory of matter (kmtom). thermal energy, internal energy present in a system in a state of thermodynamic equilibrium by virtue of its temperature. This happens by means of work done by kinetic. How Is Thermal Energy Kinetic.
From slideplayer.com
Warm Up 2 How are heat, temperature, and thermal energy related? ppt How Is Thermal Energy Kinetic The thermal energy is the. kinetic energy is the total energy of a particle or system. the idea of thermal energy is derived from the kinetic molecular theory of matter (kmtom). This happens by means of work done by kinetic friction, so this energy transfer can be treated in the same way as if it were transferred as. How Is Thermal Energy Kinetic.
From www.slideserve.com
PPT Chapter 6 Thermal Energy PowerPoint Presentation, free download How Is Thermal Energy Kinetic This happens by means of work done by kinetic friction, so this energy transfer can be treated in the same way as if it were transferred as heat. kinetic energy is the total energy of a particle or system. Particles can have translational, rotational,. thermal energy, internal energy present in a system in a state of thermodynamic equilibrium. How Is Thermal Energy Kinetic.
From www.slideserve.com
PPT Chapter 5 Thermochemistry PowerPoint Presentation, free download How Is Thermal Energy Kinetic Thermal energy cannot be converted to useful work as easily as the energy of systems that are not in states of thermodynamic equilibrium. thermal energy refers to the kinetic energy of randomly moving particles in a substance. The thermal energy is the. This happens by means of work done by kinetic friction, so this energy transfer can be treated. How Is Thermal Energy Kinetic.
From sciencenotes.org
What Is Energy? Energy Examples How Is Thermal Energy Kinetic the thermal energy is the total sum of the potential energies of the particles in a system. For example a ball falling has kinetic energy. kinetic energy is the total energy of a particle or system. the kinetic temperature is the variable needed for subjects like heat transfer, because it is the translational kinetic. This happens by. How Is Thermal Energy Kinetic.
From quizlet.com
Thermal, and Potential Energy Diagram Quizlet How Is Thermal Energy Kinetic Thermal energy cannot be converted to useful work as easily as the energy of systems that are not in states of thermodynamic equilibrium. For example a ball falling has kinetic energy. the system starts with kinetic energy, which goes away, replaced with thermal energy. the idea of thermal energy is derived from the kinetic molecular theory of matter. How Is Thermal Energy Kinetic.
From sciencing.com
Thermal Energy Definition, Equation, Types (w/ Diagram & Examples How Is Thermal Energy Kinetic the kinetic temperature is the variable needed for subjects like heat transfer, because it is the translational kinetic. thermal energy refers to the kinetic energy of randomly moving particles in a substance. The thermal energy is the. the idea of thermal energy is derived from the kinetic molecular theory of matter (kmtom). For example a ball falling. How Is Thermal Energy Kinetic.
From www.slideserve.com
PPT Ch 16 Thermal Energy and Heat PowerPoint Presentation, free How Is Thermal Energy Kinetic the kinetic temperature is the variable needed for subjects like heat transfer, because it is the translational kinetic. kinetic energy is the total energy of a particle or system. This happens by means of work done by kinetic friction, so this energy transfer can be treated in the same way as if it were transferred as heat. The. How Is Thermal Energy Kinetic.
From webapi.bu.edu
💌 Thermal energy energy. What Are the Differences Between How Is Thermal Energy Kinetic For example a ball falling has kinetic energy. This happens by means of work done by kinetic friction, so this energy transfer can be treated in the same way as if it were transferred as heat. thermal energy, internal energy present in a system in a state of thermodynamic equilibrium by virtue of its temperature. Thermal energy cannot be. How Is Thermal Energy Kinetic.
From engineerfix.com
What Is Energy? Definition, Examples, Equation, and FAQs How Is Thermal Energy Kinetic Thermal energy cannot be converted to useful work as easily as the energy of systems that are not in states of thermodynamic equilibrium. This happens by means of work done by kinetic friction, so this energy transfer can be treated in the same way as if it were transferred as heat. the thermal energy is the total sum of. How Is Thermal Energy Kinetic.
From www.slideshare.net
Thermal Energy And You How Is Thermal Energy Kinetic The thermal energy is the. the system starts with kinetic energy, which goes away, replaced with thermal energy. the idea of thermal energy is derived from the kinetic molecular theory of matter (kmtom). Particles can have translational, rotational,. thermal energy refers to the kinetic energy of randomly moving particles in a substance. kinetic energy is the. How Is Thermal Energy Kinetic.
From www.youtube.com
Thermodynamics Energy, Work and Heat (Animation) YouTube How Is Thermal Energy Kinetic the system starts with kinetic energy, which goes away, replaced with thermal energy. The thermal energy is the. For example a ball falling has kinetic energy. thermal energy, internal energy present in a system in a state of thermodynamic equilibrium by virtue of its temperature. the idea of thermal energy is derived from the kinetic molecular theory. How Is Thermal Energy Kinetic.
From www.pinterest.com.mx
Thermal energy anchor chart Sixth grade science, Science curriculum How Is Thermal Energy Kinetic The thermal energy is the. thermal energy refers to the kinetic energy of randomly moving particles in a substance. thermal energy, internal energy present in a system in a state of thermodynamic equilibrium by virtue of its temperature. the system starts with kinetic energy, which goes away, replaced with thermal energy. the idea of thermal energy. How Is Thermal Energy Kinetic.
From slideplayer.com
NOTES 14 Temperature & Thermal Energy ppt download How Is Thermal Energy Kinetic The thermal energy is the. thermal energy, internal energy present in a system in a state of thermodynamic equilibrium by virtue of its temperature. the kinetic temperature is the variable needed for subjects like heat transfer, because it is the translational kinetic. This happens by means of work done by kinetic friction, so this energy transfer can be. How Is Thermal Energy Kinetic.
From www.teachoo.com
Energy Definition, Formula, Examples Teachoo How Is Thermal Energy Kinetic the thermal energy is the total sum of the potential energies of the particles in a system. the system starts with kinetic energy, which goes away, replaced with thermal energy. Thermal energy cannot be converted to useful work as easily as the energy of systems that are not in states of thermodynamic equilibrium. thermal energy refers to. How Is Thermal Energy Kinetic.
From slideplayer.com
Transferring Thermal Energy ppt download How Is Thermal Energy Kinetic thermal energy, internal energy present in a system in a state of thermodynamic equilibrium by virtue of its temperature. The thermal energy is the. kinetic energy is the total energy of a particle or system. the kinetic temperature is the variable needed for subjects like heat transfer, because it is the translational kinetic. the thermal energy. How Is Thermal Energy Kinetic.
From www.slideshare.net
Lesson 1 thermal energy How Is Thermal Energy Kinetic For example a ball falling has kinetic energy. thermal energy, internal energy present in a system in a state of thermodynamic equilibrium by virtue of its temperature. kinetic energy is the total energy of a particle or system. thermal energy refers to the kinetic energy of randomly moving particles in a substance. Thermal energy cannot be converted. How Is Thermal Energy Kinetic.
From www.sciencefacts.net
Thermal (Heat) Energy Definition, Examples, Equations, and Units How Is Thermal Energy Kinetic Thermal energy cannot be converted to useful work as easily as the energy of systems that are not in states of thermodynamic equilibrium. This happens by means of work done by kinetic friction, so this energy transfer can be treated in the same way as if it were transferred as heat. thermal energy, internal energy present in a system. How Is Thermal Energy Kinetic.
From slideplayer.com
Thermal Energy and Heat ppt download How Is Thermal Energy Kinetic Particles can have translational, rotational,. This happens by means of work done by kinetic friction, so this energy transfer can be treated in the same way as if it were transferred as heat. the idea of thermal energy is derived from the kinetic molecular theory of matter (kmtom). Thermal energy cannot be converted to useful work as easily as. How Is Thermal Energy Kinetic.
From www.slideserve.com
PPT Chapter 10 Thermal Physics PowerPoint Presentation, free download How Is Thermal Energy Kinetic the idea of thermal energy is derived from the kinetic molecular theory of matter (kmtom). This happens by means of work done by kinetic friction, so this energy transfer can be treated in the same way as if it were transferred as heat. For example a ball falling has kinetic energy. Particles can have translational, rotational,. thermal energy,. How Is Thermal Energy Kinetic.
From courses.lumenlearning.com
Introduction to Thermodynamics Boundless Chemistry How Is Thermal Energy Kinetic the kinetic temperature is the variable needed for subjects like heat transfer, because it is the translational kinetic. Thermal energy cannot be converted to useful work as easily as the energy of systems that are not in states of thermodynamic equilibrium. kinetic energy is the total energy of a particle or system. For example a ball falling has. How Is Thermal Energy Kinetic.
From www.slideserve.com
PPT Heat, Thermodynamics, Heat Transfer and Weather PowerPoint How Is Thermal Energy Kinetic the thermal energy is the total sum of the potential energies of the particles in a system. Particles can have translational, rotational,. This happens by means of work done by kinetic friction, so this energy transfer can be treated in the same way as if it were transferred as heat. the system starts with kinetic energy, which goes. How Is Thermal Energy Kinetic.
From www.slideserve.com
PPT Energy, Work, Power PowerPoint Presentation, free download ID How Is Thermal Energy Kinetic the thermal energy is the total sum of the potential energies of the particles in a system. This happens by means of work done by kinetic friction, so this energy transfer can be treated in the same way as if it were transferred as heat. Thermal energy cannot be converted to useful work as easily as the energy of. How Is Thermal Energy Kinetic.
From www.haikudeck.com
Thermal Energy by Chaeli Norwood How Is Thermal Energy Kinetic thermal energy refers to the kinetic energy of randomly moving particles in a substance. kinetic energy is the total energy of a particle or system. the thermal energy is the total sum of the potential energies of the particles in a system. the system starts with kinetic energy, which goes away, replaced with thermal energy. This. How Is Thermal Energy Kinetic.
From exorkgevk.blob.core.windows.net
Can Energy Be Converted To Thermal Energy at John Sollars blog How Is Thermal Energy Kinetic the thermal energy is the total sum of the potential energies of the particles in a system. thermal energy refers to the kinetic energy of randomly moving particles in a substance. thermal energy, internal energy present in a system in a state of thermodynamic equilibrium by virtue of its temperature. For example a ball falling has kinetic. How Is Thermal Energy Kinetic.
From www.slideserve.com
PPT Thermal Energy A. Temperature & Heat 1. Temperature is related to How Is Thermal Energy Kinetic the kinetic temperature is the variable needed for subjects like heat transfer, because it is the translational kinetic. Particles can have translational, rotational,. thermal energy refers to the kinetic energy of randomly moving particles in a substance. Thermal energy cannot be converted to useful work as easily as the energy of systems that are not in states of. How Is Thermal Energy Kinetic.
From haipernews.com
How To Calculate Energy Thermal Haiper How Is Thermal Energy Kinetic For example a ball falling has kinetic energy. thermal energy, internal energy present in a system in a state of thermodynamic equilibrium by virtue of its temperature. the thermal energy is the total sum of the potential energies of the particles in a system. kinetic energy is the total energy of a particle or system. Particles can. How Is Thermal Energy Kinetic.
From successimg.com
Thermal Energy Examples for Kids How Is Thermal Energy Kinetic thermal energy refers to the kinetic energy of randomly moving particles in a substance. the idea of thermal energy is derived from the kinetic molecular theory of matter (kmtom). the kinetic temperature is the variable needed for subjects like heat transfer, because it is the translational kinetic. kinetic energy is the total energy of a particle. How Is Thermal Energy Kinetic.
From engineerfix.com
What is energy? Examples and some commonly asked questions How Is Thermal Energy Kinetic For example a ball falling has kinetic energy. the system starts with kinetic energy, which goes away, replaced with thermal energy. The thermal energy is the. This happens by means of work done by kinetic friction, so this energy transfer can be treated in the same way as if it were transferred as heat. thermal energy refers to. How Is Thermal Energy Kinetic.
From energyeducation.ca
Thermal conduction Energy Education How Is Thermal Energy Kinetic the idea of thermal energy is derived from the kinetic molecular theory of matter (kmtom). The thermal energy is the. Thermal energy cannot be converted to useful work as easily as the energy of systems that are not in states of thermodynamic equilibrium. the system starts with kinetic energy, which goes away, replaced with thermal energy. Particles can. How Is Thermal Energy Kinetic.
From www.yourdictionary.com
Energy Examples YourDictionary How Is Thermal Energy Kinetic thermal energy, internal energy present in a system in a state of thermodynamic equilibrium by virtue of its temperature. the system starts with kinetic energy, which goes away, replaced with thermal energy. This happens by means of work done by kinetic friction, so this energy transfer can be treated in the same way as if it were transferred. How Is Thermal Energy Kinetic.
From slideplayer.com
What are potential and energy? ppt download How Is Thermal Energy Kinetic the kinetic temperature is the variable needed for subjects like heat transfer, because it is the translational kinetic. thermal energy refers to the kinetic energy of randomly moving particles in a substance. Particles can have translational, rotational,. kinetic energy is the total energy of a particle or system. Thermal energy cannot be converted to useful work as. How Is Thermal Energy Kinetic.
From study.com
Thermal Energy Definition & Examples Lesson How Is Thermal Energy Kinetic This happens by means of work done by kinetic friction, so this energy transfer can be treated in the same way as if it were transferred as heat. thermal energy refers to the kinetic energy of randomly moving particles in a substance. the thermal energy is the total sum of the potential energies of the particles in a. How Is Thermal Energy Kinetic.
From www.slideserve.com
PPT Heat PowerPoint Presentation, free download ID2977447 How Is Thermal Energy Kinetic the system starts with kinetic energy, which goes away, replaced with thermal energy. Thermal energy cannot be converted to useful work as easily as the energy of systems that are not in states of thermodynamic equilibrium. thermal energy, internal energy present in a system in a state of thermodynamic equilibrium by virtue of its temperature. the thermal. How Is Thermal Energy Kinetic.
From www.slideserve.com
PPT Chapter 6 Thermochemistry PowerPoint Presentation, free download How Is Thermal Energy Kinetic the thermal energy is the total sum of the potential energies of the particles in a system. Thermal energy cannot be converted to useful work as easily as the energy of systems that are not in states of thermodynamic equilibrium. thermal energy, internal energy present in a system in a state of thermodynamic equilibrium by virtue of its. How Is Thermal Energy Kinetic.