Why Is Iron's Atomic Number 26 . — learn about iron, a common and essential element with the symbol fe and atomic number 26. the latin name for iron is ferrum, which is the source of its atomic symbol, fe atomic number 26; iron is a transition metal with the symbol fe and atomic number 26. It is the most abundant element in earth and has many. Find out its properties, uses, isotopes, allotropes, and more. The number of neutrons, however, can vary. Iron has four stable isotopes, and each one has a different number of neutrons. — iron is a metal of group 8 with atomic number 26 and atomic weight 55.847. It is widely used in alloys such as steel and has many applications in industry,.
from utedzz.blogspot.com
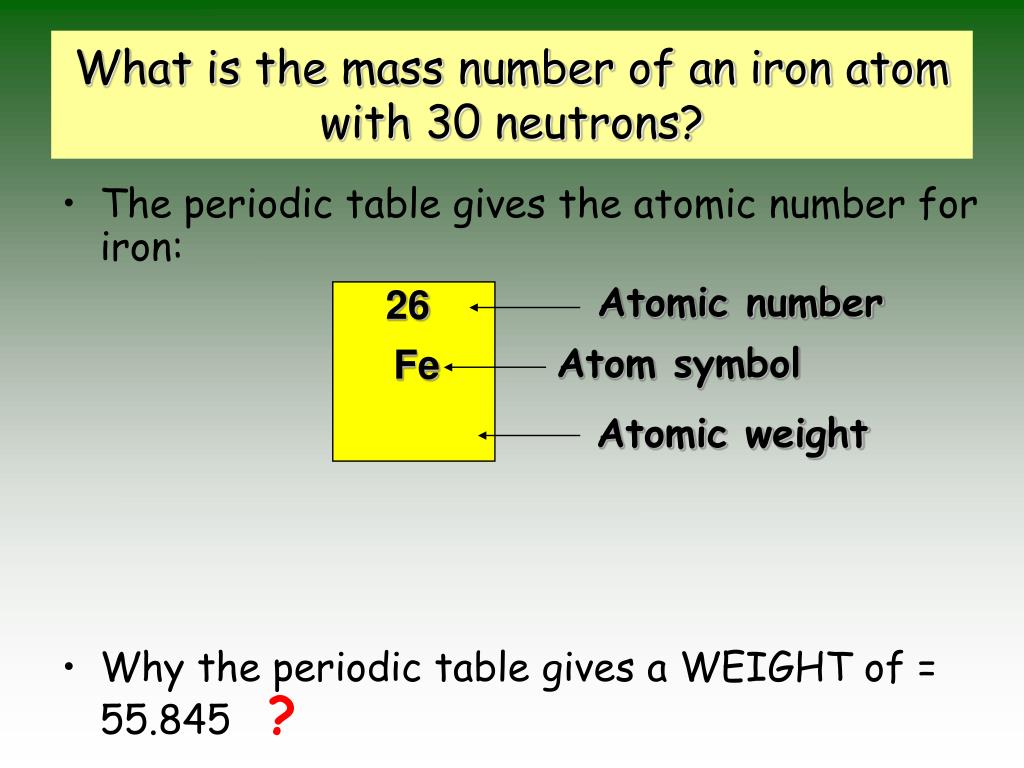
— iron is a metal of group 8 with atomic number 26 and atomic weight 55.847. Iron has four stable isotopes, and each one has a different number of neutrons. The number of neutrons, however, can vary. Find out its properties, uses, isotopes, allotropes, and more. iron is a transition metal with the symbol fe and atomic number 26. the latin name for iron is ferrum, which is the source of its atomic symbol, fe atomic number 26; — learn about iron, a common and essential element with the symbol fe and atomic number 26. It is widely used in alloys such as steel and has many applications in industry,. It is the most abundant element in earth and has many.
Periodic Table Iron Atomic Mass Periodic Table Timeline
Why Is Iron's Atomic Number 26 Iron has four stable isotopes, and each one has a different number of neutrons. — learn about iron, a common and essential element with the symbol fe and atomic number 26. the latin name for iron is ferrum, which is the source of its atomic symbol, fe atomic number 26; iron is a transition metal with the symbol fe and atomic number 26. The number of neutrons, however, can vary. — iron is a metal of group 8 with atomic number 26 and atomic weight 55.847. It is the most abundant element in earth and has many. It is widely used in alloys such as steel and has many applications in industry,. Find out its properties, uses, isotopes, allotropes, and more. Iron has four stable isotopes, and each one has a different number of neutrons.
From www.youtube.com
Element 26 Iron facts YouTube Why Is Iron's Atomic Number 26 The number of neutrons, however, can vary. — learn about iron, a common and essential element with the symbol fe and atomic number 26. Find out its properties, uses, isotopes, allotropes, and more. the latin name for iron is ferrum, which is the source of its atomic symbol, fe atomic number 26; It is widely used in alloys. Why Is Iron's Atomic Number 26.
From www.alamy.com
Iron symbol. Element number 26 of the Periodic Table of the Elements Why Is Iron's Atomic Number 26 Iron has four stable isotopes, and each one has a different number of neutrons. The number of neutrons, however, can vary. It is the most abundant element in earth and has many. — learn about iron, a common and essential element with the symbol fe and atomic number 26. Find out its properties, uses, isotopes, allotropes, and more. . Why Is Iron's Atomic Number 26.
From utedzz.blogspot.com
Periodic Table Iron Atomic Mass Periodic Table Timeline Why Is Iron's Atomic Number 26 Find out its properties, uses, isotopes, allotropes, and more. It is widely used in alloys such as steel and has many applications in industry,. — iron is a metal of group 8 with atomic number 26 and atomic weight 55.847. iron is a transition metal with the symbol fe and atomic number 26. the latin name for. Why Is Iron's Atomic Number 26.
From www.slideserve.com
PPT Physical Science Vocabulary PowerPoint Presentation, free Why Is Iron's Atomic Number 26 It is the most abundant element in earth and has many. Iron has four stable isotopes, and each one has a different number of neutrons. the latin name for iron is ferrum, which is the source of its atomic symbol, fe atomic number 26; Find out its properties, uses, isotopes, allotropes, and more. It is widely used in alloys. Why Is Iron's Atomic Number 26.
From www.asbmb.org
For February, it’s iron — atomic No. 26 Why Is Iron's Atomic Number 26 It is widely used in alloys such as steel and has many applications in industry,. iron is a transition metal with the symbol fe and atomic number 26. Iron has four stable isotopes, and each one has a different number of neutrons. — learn about iron, a common and essential element with the symbol fe and atomic number. Why Is Iron's Atomic Number 26.
From www.nuclear-power.com
Iron Atomic Number Atomic Mass Density of Iron Why Is Iron's Atomic Number 26 Find out its properties, uses, isotopes, allotropes, and more. — iron is a metal of group 8 with atomic number 26 and atomic weight 55.847. It is widely used in alloys such as steel and has many applications in industry,. The number of neutrons, however, can vary. Iron has four stable isotopes, and each one has a different number. Why Is Iron's Atomic Number 26.
From www.alamy.com
Iron chemical element, Sign with atomic number and atomic weight Why Is Iron's Atomic Number 26 — iron is a metal of group 8 with atomic number 26 and atomic weight 55.847. Iron has four stable isotopes, and each one has a different number of neutrons. It is the most abundant element in earth and has many. — learn about iron, a common and essential element with the symbol fe and atomic number 26.. Why Is Iron's Atomic Number 26.
From www.youtube.com
Atomic number of Iron Iron atomic number What is atomic number of Why Is Iron's Atomic Number 26 the latin name for iron is ferrum, which is the source of its atomic symbol, fe atomic number 26; iron is a transition metal with the symbol fe and atomic number 26. It is the most abundant element in earth and has many. Iron has four stable isotopes, and each one has a different number of neutrons. The. Why Is Iron's Atomic Number 26.
From dxopgqpsw.blob.core.windows.net
Iron Electron Density at Arlyne Henry blog Why Is Iron's Atomic Number 26 Iron has four stable isotopes, and each one has a different number of neutrons. It is the most abundant element in earth and has many. — iron is a metal of group 8 with atomic number 26 and atomic weight 55.847. the latin name for iron is ferrum, which is the source of its atomic symbol, fe atomic. Why Is Iron's Atomic Number 26.
From ar.inspiredpencil.com
Iron Atomic Structure Why Is Iron's Atomic Number 26 the latin name for iron is ferrum, which is the source of its atomic symbol, fe atomic number 26; It is widely used in alloys such as steel and has many applications in industry,. — iron is a metal of group 8 with atomic number 26 and atomic weight 55.847. Iron has four stable isotopes, and each one. Why Is Iron's Atomic Number 26.
From docslib.org
Iron Is a Chemical Element with Atomic Number 26. Iron Is a Lustrous Why Is Iron's Atomic Number 26 It is widely used in alloys such as steel and has many applications in industry,. iron is a transition metal with the symbol fe and atomic number 26. The number of neutrons, however, can vary. Find out its properties, uses, isotopes, allotropes, and more. the latin name for iron is ferrum, which is the source of its atomic. Why Is Iron's Atomic Number 26.
From in.pinterest.com
Iron was discovered before 5000 BC. Iron is a chemical element with Why Is Iron's Atomic Number 26 — learn about iron, a common and essential element with the symbol fe and atomic number 26. — iron is a metal of group 8 with atomic number 26 and atomic weight 55.847. Find out its properties, uses, isotopes, allotropes, and more. Iron has four stable isotopes, and each one has a different number of neutrons. It is. Why Is Iron's Atomic Number 26.
From www.dreamstime.com
Periodic Table of Elements Iron Stock Illustration Illustration of Why Is Iron's Atomic Number 26 — learn about iron, a common and essential element with the symbol fe and atomic number 26. Find out its properties, uses, isotopes, allotropes, and more. It is widely used in alloys such as steel and has many applications in industry,. It is the most abundant element in earth and has many. iron is a transition metal with. Why Is Iron's Atomic Number 26.
From stock.adobe.com
Iron atomic structure has atomic number, atomic mass, electron Why Is Iron's Atomic Number 26 It is the most abundant element in earth and has many. Iron has four stable isotopes, and each one has a different number of neutrons. the latin name for iron is ferrum, which is the source of its atomic symbol, fe atomic number 26; — iron is a metal of group 8 with atomic number 26 and atomic. Why Is Iron's Atomic Number 26.
From www.alamy.com
Iron symbol. Element number 26 of the Periodic Table of the Elements Why Is Iron's Atomic Number 26 The number of neutrons, however, can vary. It is widely used in alloys such as steel and has many applications in industry,. It is the most abundant element in earth and has many. Iron has four stable isotopes, and each one has a different number of neutrons. Find out its properties, uses, isotopes, allotropes, and more. the latin name. Why Is Iron's Atomic Number 26.
From www.dreamstime.com
Iron Fe Chemical Element. Iron Sign with Atomic Number. Chemical 26 Why Is Iron's Atomic Number 26 It is widely used in alloys such as steel and has many applications in industry,. Find out its properties, uses, isotopes, allotropes, and more. It is the most abundant element in earth and has many. the latin name for iron is ferrum, which is the source of its atomic symbol, fe atomic number 26; The number of neutrons, however,. Why Is Iron's Atomic Number 26.
From www.alamy.com
Atomic number 26 hires stock photography and images Alamy Why Is Iron's Atomic Number 26 It is the most abundant element in earth and has many. the latin name for iron is ferrum, which is the source of its atomic symbol, fe atomic number 26; Iron has four stable isotopes, and each one has a different number of neutrons. — iron is a metal of group 8 with atomic number 26 and atomic. Why Is Iron's Atomic Number 26.
From chemistry291.blogspot.com
【5 Steps】Electron Configuration of Iron(Fe) Electron configuration Why Is Iron's Atomic Number 26 Iron has four stable isotopes, and each one has a different number of neutrons. It is widely used in alloys such as steel and has many applications in industry,. It is the most abundant element in earth and has many. Find out its properties, uses, isotopes, allotropes, and more. the latin name for iron is ferrum, which is the. Why Is Iron's Atomic Number 26.
From www.freepik.com
Premium Photo Iron chemical element, sign with atomic number and Why Is Iron's Atomic Number 26 The number of neutrons, however, can vary. It is widely used in alloys such as steel and has many applications in industry,. — iron is a metal of group 8 with atomic number 26 and atomic weight 55.847. Find out its properties, uses, isotopes, allotropes, and more. the latin name for iron is ferrum, which is the source. Why Is Iron's Atomic Number 26.
From www.wisegeek.com
What is an Atomic Number? (with pictures) Why Is Iron's Atomic Number 26 Find out its properties, uses, isotopes, allotropes, and more. It is widely used in alloys such as steel and has many applications in industry,. — iron is a metal of group 8 with atomic number 26 and atomic weight 55.847. the latin name for iron is ferrum, which is the source of its atomic symbol, fe atomic number. Why Is Iron's Atomic Number 26.
From www.alamy.com
Diagram of the nuclear composition, electron configuration, and valence Why Is Iron's Atomic Number 26 Find out its properties, uses, isotopes, allotropes, and more. Iron has four stable isotopes, and each one has a different number of neutrons. the latin name for iron is ferrum, which is the source of its atomic symbol, fe atomic number 26; iron is a transition metal with the symbol fe and atomic number 26. — iron. Why Is Iron's Atomic Number 26.
From www.sciencephoto.com
Iron, atomic structure Stock Image C018/3707 Science Photo Library Why Is Iron's Atomic Number 26 It is widely used in alloys such as steel and has many applications in industry,. — iron is a metal of group 8 with atomic number 26 and atomic weight 55.847. The number of neutrons, however, can vary. — learn about iron, a common and essential element with the symbol fe and atomic number 26. the latin. Why Is Iron's Atomic Number 26.
From sciencenotes.org
Iron Facts Atomic Number 26 or Fe Why Is Iron's Atomic Number 26 Find out its properties, uses, isotopes, allotropes, and more. iron is a transition metal with the symbol fe and atomic number 26. Iron has four stable isotopes, and each one has a different number of neutrons. the latin name for iron is ferrum, which is the source of its atomic symbol, fe atomic number 26; The number of. Why Is Iron's Atomic Number 26.
From www.alamy.com
Iron (Fe). Diagram of the nuclear composition and electron Why Is Iron's Atomic Number 26 the latin name for iron is ferrum, which is the source of its atomic symbol, fe atomic number 26; Find out its properties, uses, isotopes, allotropes, and more. — learn about iron, a common and essential element with the symbol fe and atomic number 26. iron is a transition metal with the symbol fe and atomic number. Why Is Iron's Atomic Number 26.
From www.alamy.com
Iron symbol on chemical flask. Element number 26 of the Periodic Table Why Is Iron's Atomic Number 26 — learn about iron, a common and essential element with the symbol fe and atomic number 26. It is the most abundant element in earth and has many. — iron is a metal of group 8 with atomic number 26 and atomic weight 55.847. It is widely used in alloys such as steel and has many applications in. Why Is Iron's Atomic Number 26.
From animalia-life.club
Iron Symbol Periodic Table Why Is Iron's Atomic Number 26 — learn about iron, a common and essential element with the symbol fe and atomic number 26. It is widely used in alloys such as steel and has many applications in industry,. It is the most abundant element in earth and has many. the latin name for iron is ferrum, which is the source of its atomic symbol,. Why Is Iron's Atomic Number 26.
From www.animalia-life.club
Iron Element Symbol Black And White Why Is Iron's Atomic Number 26 iron is a transition metal with the symbol fe and atomic number 26. — learn about iron, a common and essential element with the symbol fe and atomic number 26. — iron is a metal of group 8 with atomic number 26 and atomic weight 55.847. Iron has four stable isotopes, and each one has a different. Why Is Iron's Atomic Number 26.
From www.truth-seeker.info
The Atomic Number of Iron Why Is Iron's Atomic Number 26 It is the most abundant element in earth and has many. It is widely used in alloys such as steel and has many applications in industry,. The number of neutrons, however, can vary. Iron has four stable isotopes, and each one has a different number of neutrons. — iron is a metal of group 8 with atomic number 26. Why Is Iron's Atomic Number 26.
From material-properties.org
Iron Periodic Table and Atomic Properties Why Is Iron's Atomic Number 26 iron is a transition metal with the symbol fe and atomic number 26. Iron has four stable isotopes, and each one has a different number of neutrons. — iron is a metal of group 8 with atomic number 26 and atomic weight 55.847. the latin name for iron is ferrum, which is the source of its atomic. Why Is Iron's Atomic Number 26.
From www.youtube.com
Which element has the atomic number 26 YouTube Why Is Iron's Atomic Number 26 iron is a transition metal with the symbol fe and atomic number 26. the latin name for iron is ferrum, which is the source of its atomic symbol, fe atomic number 26; — learn about iron, a common and essential element with the symbol fe and atomic number 26. Find out its properties, uses, isotopes, allotropes, and. Why Is Iron's Atomic Number 26.
From tankhrom.weebly.com
Iron atomic number tankhrom Why Is Iron's Atomic Number 26 Iron has four stable isotopes, and each one has a different number of neutrons. The number of neutrons, however, can vary. the latin name for iron is ferrum, which is the source of its atomic symbol, fe atomic number 26; It is widely used in alloys such as steel and has many applications in industry,. It is the most. Why Is Iron's Atomic Number 26.
From www.breakingatom.com
Iron (Fe) Atomic Number 26 Why Is Iron's Atomic Number 26 — iron is a metal of group 8 with atomic number 26 and atomic weight 55.847. iron is a transition metal with the symbol fe and atomic number 26. — learn about iron, a common and essential element with the symbol fe and atomic number 26. Find out its properties, uses, isotopes, allotropes, and more. It is. Why Is Iron's Atomic Number 26.
From www.alamy.com
Iron chemical element, Sign with atomic number and atomic weight Why Is Iron's Atomic Number 26 The number of neutrons, however, can vary. the latin name for iron is ferrum, which is the source of its atomic symbol, fe atomic number 26; It is widely used in alloys such as steel and has many applications in industry,. Iron has four stable isotopes, and each one has a different number of neutrons. — iron is. Why Is Iron's Atomic Number 26.
From www.alamy.com
Iron element on the periodic table. transition metal Why Is Iron's Atomic Number 26 iron is a transition metal with the symbol fe and atomic number 26. Find out its properties, uses, isotopes, allotropes, and more. the latin name for iron is ferrum, which is the source of its atomic symbol, fe atomic number 26; Iron has four stable isotopes, and each one has a different number of neutrons. — iron. Why Is Iron's Atomic Number 26.
From sciencenotes.org
Iron Facts Atomic Number 26 or Fe Why Is Iron's Atomic Number 26 It is widely used in alloys such as steel and has many applications in industry,. the latin name for iron is ferrum, which is the source of its atomic symbol, fe atomic number 26; — iron is a metal of group 8 with atomic number 26 and atomic weight 55.847. Find out its properties, uses, isotopes, allotropes, and. Why Is Iron's Atomic Number 26.