How To Find Concentration Using Titration Data . the process of calculating concentration from titration data is described and illustrated. C1v1 = c2v2 where c1 is the concentration of the titrant (the. titration is a laboratory technique used to determine the concentration of a solution by reacting it with a known volume and concentration of another. in a titration, a carefully measured volume of a solution of known concentration, called the titrant, is added to a measured volume of a. to calculate concentration from titration, you can use the formula: M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. a titration calculation is a simple formula used to work out the concentration (in moles) of one of the reactants in a titration using the.
from quizpseudology.z21.web.core.windows.net
C1v1 = c2v2 where c1 is the concentration of the titrant (the. a titration calculation is a simple formula used to work out the concentration (in moles) of one of the reactants in a titration using the. the process of calculating concentration from titration data is described and illustrated. M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration. titration is a laboratory technique used to determine the concentration of a solution by reacting it with a known volume and concentration of another. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. to calculate concentration from titration, you can use the formula: in a titration, a carefully measured volume of a solution of known concentration, called the titrant, is added to a measured volume of a.
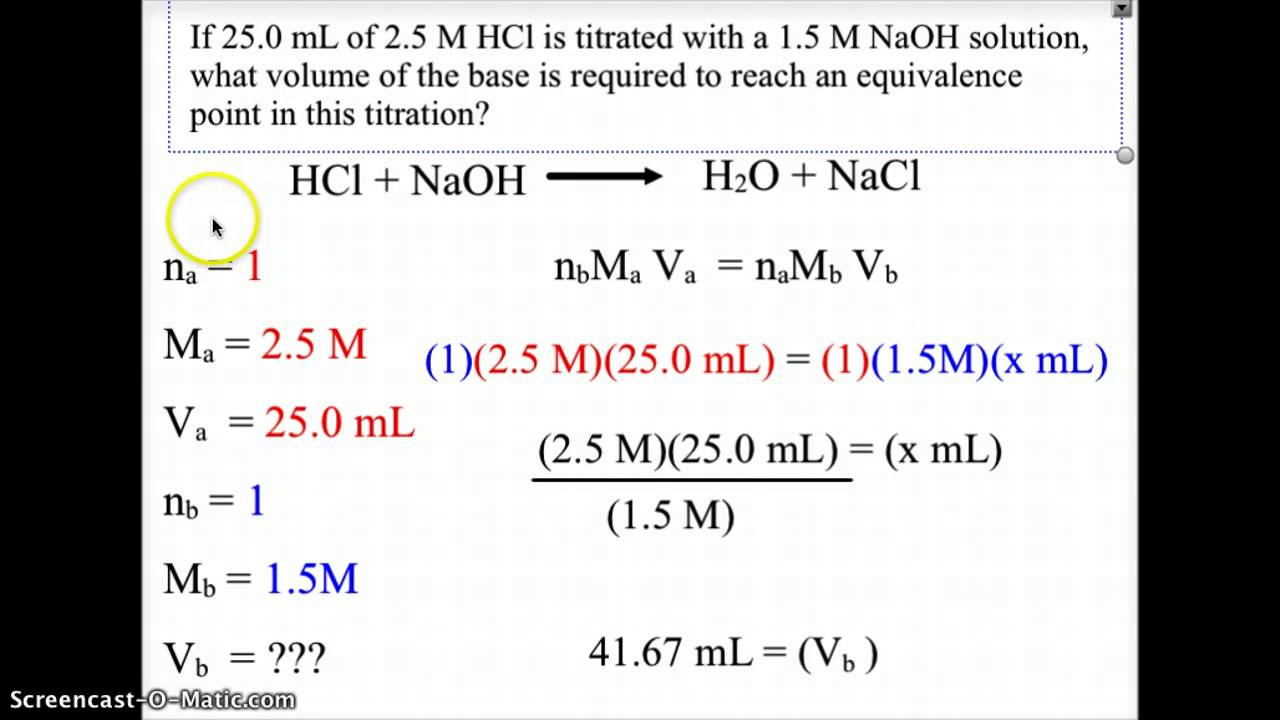
How To Find Equivalence Point Volume
How To Find Concentration Using Titration Data to calculate concentration from titration, you can use the formula: M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration. titration is a laboratory technique used to determine the concentration of a solution by reacting it with a known volume and concentration of another. in a titration, a carefully measured volume of a solution of known concentration, called the titrant, is added to a measured volume of a. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. the process of calculating concentration from titration data is described and illustrated. C1v1 = c2v2 where c1 is the concentration of the titrant (the. a titration calculation is a simple formula used to work out the concentration (in moles) of one of the reactants in a titration using the. to calculate concentration from titration, you can use the formula:
From www.youtube.com
CHEM 101 Titration of Unknown Triprotic Acid YouTube How To Find Concentration Using Titration Data C1v1 = c2v2 where c1 is the concentration of the titrant (the. M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration. in a titration, a carefully measured volume of a solution of known concentration, called the titrant, is added to a measured volume of a. the process of calculating concentration from titration. How To Find Concentration Using Titration Data.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation How To Find Concentration Using Titration Data in a titration, a carefully measured volume of a solution of known concentration, called the titrant, is added to a measured volume of a. titration is a laboratory technique used to determine the concentration of a solution by reacting it with a known volume and concentration of another. In a titration, 25.0 cm 3 of 0.100 mol/dm 3. How To Find Concentration Using Titration Data.
From dxokhzpxh.blob.core.windows.net
Aim Of Acid Base Titration Experiment at Ebony Mooney blog How To Find Concentration Using Titration Data to calculate concentration from titration, you can use the formula: M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration. in a titration, a carefully measured volume of a solution of known concentration, called the titrant, is added to a measured volume of a. the process of calculating concentration from titration data. How To Find Concentration Using Titration Data.
From learningzonelisannin.z14.web.core.windows.net
Chemistry Titration Questions And Answers How To Find Concentration Using Titration Data M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration. the process of calculating concentration from titration data is described and illustrated. a titration calculation is a simple formula used to work out the concentration (in moles) of one of the reactants in a titration using the. in a titration, a carefully. How To Find Concentration Using Titration Data.
From learningschoolmurgkerny1.z13.web.core.windows.net
Titration Practical Questions And Answers Pdf How To Find Concentration Using Titration Data titration is a laboratory technique used to determine the concentration of a solution by reacting it with a known volume and concentration of another. a titration calculation is a simple formula used to work out the concentration (in moles) of one of the reactants in a titration using the. M_av_a = m_bv_b let's assume you are titrating a. How To Find Concentration Using Titration Data.
From www.chegg.com
Solved TITRATION ⋅ STANDARDIZATION OF SODIUM HYDROXIDE How To Find Concentration Using Titration Data In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. titration is a laboratory technique used to determine the concentration of a solution by reacting it with a known volume and concentration of another. a titration calculation is a simple formula used to work out the concentration (in moles) of one of the reactants. How To Find Concentration Using Titration Data.
From www.youtube.com
titration curve pH calculations YouTube How To Find Concentration Using Titration Data M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration. the process of calculating concentration from titration data is described and illustrated. a titration calculation is a simple formula used to work out the concentration (in moles) of one of the reactants in a titration using the. titration is a laboratory technique. How To Find Concentration Using Titration Data.
From studyfinder.org
Unlocking the Mysteries Titration of Acids and Bases Lab Answers Revealed How To Find Concentration Using Titration Data M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration. C1v1 = c2v2 where c1 is the concentration of the titrant (the. the process of calculating concentration from titration data is described and illustrated. titration is a laboratory technique used to determine the concentration of a solution by reacting it with a known. How To Find Concentration Using Titration Data.
From quizpseudology.z21.web.core.windows.net
How To Find Equivalence Point Volume How To Find Concentration Using Titration Data to calculate concentration from titration, you can use the formula: a titration calculation is a simple formula used to work out the concentration (in moles) of one of the reactants in a titration using the. titration is a laboratory technique used to determine the concentration of a solution by reacting it with a known volume and concentration. How To Find Concentration Using Titration Data.
From exommzgkk.blob.core.windows.net
Titration Concentration Of Solution at Tony Pelletier blog How To Find Concentration Using Titration Data to calculate concentration from titration, you can use the formula: M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration. C1v1 = c2v2 where c1 is the concentration of the titrant (the. in a titration, a carefully measured volume of a solution of known concentration, called the titrant, is added to a measured. How To Find Concentration Using Titration Data.
From exosjwdrb.blob.core.windows.net
Titration Calculation Table Method at Susan Clyburn blog How To Find Concentration Using Titration Data in a titration, a carefully measured volume of a solution of known concentration, called the titrant, is added to a measured volume of a. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. the process of calculating concentration from titration data is described and illustrated. C1v1 = c2v2 where c1 is the concentration. How To Find Concentration Using Titration Data.
From www.youtube.com
Titration of an unknown acid with a standardized Sodium Hydroxide How To Find Concentration Using Titration Data the process of calculating concentration from titration data is described and illustrated. M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration. titration is a laboratory technique used to determine the concentration of a solution by reacting it with a known volume and concentration of another. a titration calculation is a simple. How To Find Concentration Using Titration Data.
From quizpseudology.z21.web.core.windows.net
How To Find Equivalence Point On Excel Graph How To Find Concentration Using Titration Data the process of calculating concentration from titration data is described and illustrated. to calculate concentration from titration, you can use the formula: M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration. in a titration, a carefully measured volume of a solution of known concentration, called the titrant, is added to a. How To Find Concentration Using Titration Data.
From dxooyumci.blob.core.windows.net
Titration Practice Problems Chemistry at Gloria Ogorman blog How To Find Concentration Using Titration Data titration is a laboratory technique used to determine the concentration of a solution by reacting it with a known volume and concentration of another. the process of calculating concentration from titration data is described and illustrated. in a titration, a carefully measured volume of a solution of known concentration, called the titrant, is added to a measured. How To Find Concentration Using Titration Data.
From dxonsvczs.blob.core.windows.net
Titration Base Concentration at James Cummings blog How To Find Concentration Using Titration Data in a titration, a carefully measured volume of a solution of known concentration, called the titrant, is added to a measured volume of a. to calculate concentration from titration, you can use the formula: a titration calculation is a simple formula used to work out the concentration (in moles) of one of the reactants in a titration. How To Find Concentration Using Titration Data.
From www.youtube.com
Finding concentration of acid or base given amount needed to titrate How To Find Concentration Using Titration Data a titration calculation is a simple formula used to work out the concentration (in moles) of one of the reactants in a titration using the. the process of calculating concentration from titration data is described and illustrated. C1v1 = c2v2 where c1 is the concentration of the titrant (the. In a titration, 25.0 cm 3 of 0.100 mol/dm. How To Find Concentration Using Titration Data.
From www.youtube.com
The titration of 25 0 mL of an unknown concentration of H2SO4 solution How To Find Concentration Using Titration Data In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. a titration calculation is a simple formula used to work out the concentration (in moles) of one of the reactants in a titration using the. C1v1 = c2v2 where c1 is the concentration of the titrant (the. in a titration, a carefully measured volume. How To Find Concentration Using Titration Data.
From www.youtube.com
Stoichiometry Problem Titration Calculation YouTube How To Find Concentration Using Titration Data In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration. C1v1 = c2v2 where c1 is the concentration of the titrant (the. a titration calculation is a simple formula used to work out the concentration (in moles) of one of. How To Find Concentration Using Titration Data.
From exokpafwp.blob.core.windows.net
Titration Endpoint Calculator at Kevin Dowell blog How To Find Concentration Using Titration Data M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration. the process of calculating concentration from titration data is described and illustrated. in a titration, a carefully measured volume of a solution of known concentration, called the titrant, is added to a measured volume of a. to calculate concentration from titration, you. How To Find Concentration Using Titration Data.
From chemistry.stackexchange.com
acid base Does diluting the titration flask change the molarity How To Find Concentration Using Titration Data a titration calculation is a simple formula used to work out the concentration (in moles) of one of the reactants in a titration using the. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. titration is a laboratory technique used to determine the concentration of a solution by reacting it with a known. How To Find Concentration Using Titration Data.
From dxopqqjgm.blob.core.windows.net
Titration Concentration Formula at Stephen Hansen blog How To Find Concentration Using Titration Data C1v1 = c2v2 where c1 is the concentration of the titrant (the. in a titration, a carefully measured volume of a solution of known concentration, called the titrant, is added to a measured volume of a. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. a titration calculation is a simple formula used. How To Find Concentration Using Titration Data.
From www.youtube.com
Worked example Determining solute concentration by acidbase titration How To Find Concentration Using Titration Data C1v1 = c2v2 where c1 is the concentration of the titrant (the. in a titration, a carefully measured volume of a solution of known concentration, called the titrant, is added to a measured volume of a. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. a titration calculation is a simple formula used. How To Find Concentration Using Titration Data.
From dxolxlskr.blob.core.windows.net
Why Use Titration To Find Concentration at Rodney Ruggerio blog How To Find Concentration Using Titration Data a titration calculation is a simple formula used to work out the concentration (in moles) of one of the reactants in a titration using the. to calculate concentration from titration, you can use the formula: in a titration, a carefully measured volume of a solution of known concentration, called the titrant, is added to a measured volume. How To Find Concentration Using Titration Data.
From exommzgkk.blob.core.windows.net
Titration Concentration Of Solution at Tony Pelletier blog How To Find Concentration Using Titration Data M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration. titration is a laboratory technique used to determine the concentration of a solution by reacting it with a known volume and concentration of another. in a titration, a carefully measured volume of a solution of known concentration, called the titrant, is added to. How To Find Concentration Using Titration Data.
From www.easybiologyclass.com
What is Titration Curve? How Do You Find pKa? easybiologyclass How To Find Concentration Using Titration Data C1v1 = c2v2 where c1 is the concentration of the titrant (the. a titration calculation is a simple formula used to work out the concentration (in moles) of one of the reactants in a titration using the. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. the process of calculating concentration from titration. How To Find Concentration Using Titration Data.
From www.chegg.com
Solved CHEMISTRY. IDENTIFY WEAK ACID USING TITRATION CURVE A How To Find Concentration Using Titration Data M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration. to calculate concentration from titration, you can use the formula: the process of calculating concentration from titration data is described and illustrated. titration is a laboratory technique used to determine the concentration of a solution by reacting it with a known volume. How To Find Concentration Using Titration Data.
From www.youtube.com
Titration of unknown weak acid with strong base YouTube How To Find Concentration Using Titration Data the process of calculating concentration from titration data is described and illustrated. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. titration is a laboratory technique used to determine the concentration of a solution by reacting it with a known volume and concentration of another. a titration calculation is a simple formula. How To Find Concentration Using Titration Data.
From www.numerade.com
Titration for Acetic Acid in Vinegar Lab Report Exercise 1 How To Find Concentration Using Titration Data M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration. a titration calculation is a simple formula used to work out the concentration (in moles) of one of the reactants in a titration using the. titration is a laboratory technique used to determine the concentration of a solution by reacting it with a. How To Find Concentration Using Titration Data.
From www.youtube.com
Acid Base Titration Curves pH Calculations YouTube How To Find Concentration Using Titration Data M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. titration is a laboratory technique used to determine the concentration of a solution by reacting it with a known volume and concentration of another. in a titration, a carefully. How To Find Concentration Using Titration Data.
From exosjwdrb.blob.core.windows.net
Titration Calculation Table Method at Susan Clyburn blog How To Find Concentration Using Titration Data to calculate concentration from titration, you can use the formula: C1v1 = c2v2 where c1 is the concentration of the titrant (the. a titration calculation is a simple formula used to work out the concentration (in moles) of one of the reactants in a titration using the. M_av_a = m_bv_b let's assume you are titrating a strong acid. How To Find Concentration Using Titration Data.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog How To Find Concentration Using Titration Data In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. C1v1 = c2v2 where c1 is the concentration of the titrant (the. a titration calculation is a simple formula used to work out the concentration (in moles) of one of the reactants in a titration using the. to calculate concentration from titration, you can. How To Find Concentration Using Titration Data.
From worksheetdbpreif.z19.web.core.windows.net
How To Work Out The Concentration How To Find Concentration Using Titration Data M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration. the process of calculating concentration from titration data is described and illustrated. to calculate concentration from titration, you can use the formula: a titration calculation is a simple formula used to work out the concentration (in moles) of one of the reactants. How To Find Concentration Using Titration Data.
From exoccrkbm.blob.core.windows.net
Titration Definition Chemistry Simple at Mary McGee blog How To Find Concentration Using Titration Data M_av_a = m_bv_b let's assume you are titrating a strong acid (10 ml unknown concentration. a titration calculation is a simple formula used to work out the concentration (in moles) of one of the reactants in a titration using the. in a titration, a carefully measured volume of a solution of known concentration, called the titrant, is added. How To Find Concentration Using Titration Data.
From dxodkszcm.blob.core.windows.net
Titration In Chemistry Is at Laura Fraser blog How To Find Concentration Using Titration Data C1v1 = c2v2 where c1 is the concentration of the titrant (the. in a titration, a carefully measured volume of a solution of known concentration, called the titrant, is added to a measured volume of a. the process of calculating concentration from titration data is described and illustrated. to calculate concentration from titration, you can use the. How To Find Concentration Using Titration Data.
From www.reddit.com
How to find concentration from a titration curve? r/chemistryhelp How To Find Concentration Using Titration Data titration is a laboratory technique used to determine the concentration of a solution by reacting it with a known volume and concentration of another. In a titration, 25.0 cm 3 of 0.100 mol/dm 3 sodium hydroxide solution is. to calculate concentration from titration, you can use the formula: a titration calculation is a simple formula used to. How To Find Concentration Using Titration Data.