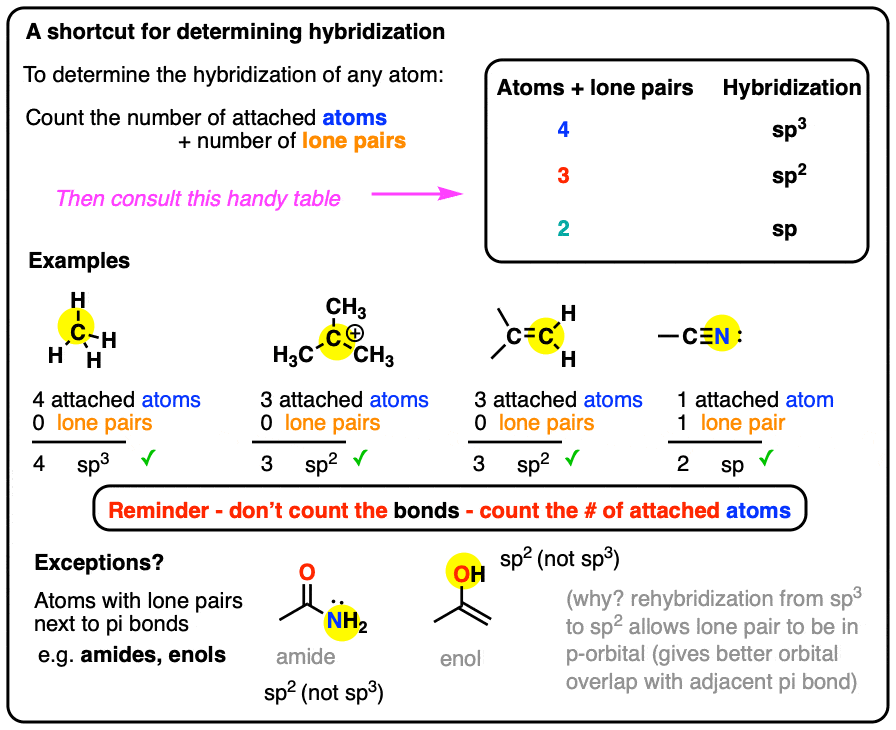
What Is The Hybridization Of N . There are 5 + 2×6 + 1 = 18 electrons, and 4 are used to make the two covalent. — hybridization is the process of combining pure atomic orbitals on an atom of approximately equal energy to form a new set of orbitals with the same number as the mixing orbitals, the same energy, and the same shape. Two of the sp 3 hybridized orbitals overlap with s. For example, sp 3 hybridization for nitrogen results in. The new orbitals produced in this way are known as hybrid orbitals. the hybridization schemes for nitrogen and oxygen follow the same guidelines as for carbon. the nitrogen is sp 3 hybridized which means that it has four sp 3 hybrid orbitals. Nitrogen is the central atom: The nitrogen atom also hybridizes in the sp 2.
from www.masterorganicchemistry.com
the hybridization schemes for nitrogen and oxygen follow the same guidelines as for carbon. There are 5 + 2×6 + 1 = 18 electrons, and 4 are used to make the two covalent. Nitrogen is the central atom: The nitrogen atom also hybridizes in the sp 2. Two of the sp 3 hybridized orbitals overlap with s. The new orbitals produced in this way are known as hybrid orbitals. For example, sp 3 hybridization for nitrogen results in. the nitrogen is sp 3 hybridized which means that it has four sp 3 hybrid orbitals. — hybridization is the process of combining pure atomic orbitals on an atom of approximately equal energy to form a new set of orbitals with the same number as the mixing orbitals, the same energy, and the same shape.
How To Determine Hybridization A Shortcut Master Organic Chemistry
What Is The Hybridization Of N the hybridization schemes for nitrogen and oxygen follow the same guidelines as for carbon. the nitrogen is sp 3 hybridized which means that it has four sp 3 hybrid orbitals. Nitrogen is the central atom: There are 5 + 2×6 + 1 = 18 electrons, and 4 are used to make the two covalent. the hybridization schemes for nitrogen and oxygen follow the same guidelines as for carbon. The nitrogen atom also hybridizes in the sp 2. The new orbitals produced in this way are known as hybrid orbitals. For example, sp 3 hybridization for nitrogen results in. Two of the sp 3 hybridized orbitals overlap with s. — hybridization is the process of combining pure atomic orbitals on an atom of approximately equal energy to form a new set of orbitals with the same number as the mixing orbitals, the same energy, and the same shape.
From ar.inspiredpencil.com
Hybridization Orbitals Chart What Is The Hybridization Of N the nitrogen is sp 3 hybridized which means that it has four sp 3 hybrid orbitals. — hybridization is the process of combining pure atomic orbitals on an atom of approximately equal energy to form a new set of orbitals with the same number as the mixing orbitals, the same energy, and the same shape. For example, sp. What Is The Hybridization Of N.
From chem.libretexts.org
Hybridization Chemistry LibreTexts What Is The Hybridization Of N — hybridization is the process of combining pure atomic orbitals on an atom of approximately equal energy to form a new set of orbitals with the same number as the mixing orbitals, the same energy, and the same shape. the nitrogen is sp 3 hybridized which means that it has four sp 3 hybrid orbitals. The nitrogen atom. What Is The Hybridization Of N.
From chem.libretexts.org
Hybridization Chemistry LibreTexts What Is The Hybridization Of N — hybridization is the process of combining pure atomic orbitals on an atom of approximately equal energy to form a new set of orbitals with the same number as the mixing orbitals, the same energy, and the same shape. Two of the sp 3 hybridized orbitals overlap with s. Nitrogen is the central atom: The nitrogen atom also hybridizes. What Is The Hybridization Of N.
From byjus.com
Hybridization of Carbon Molecular Geometry and Bond Angles What Is The Hybridization Of N Two of the sp 3 hybridized orbitals overlap with s. The new orbitals produced in this way are known as hybrid orbitals. the nitrogen is sp 3 hybridized which means that it has four sp 3 hybrid orbitals. The nitrogen atom also hybridizes in the sp 2. There are 5 + 2×6 + 1 = 18 electrons, and 4. What Is The Hybridization Of N.
From general.chemistrysteps.com
Hybridization of Atomic Orbitals Chemistry Steps What Is The Hybridization Of N The nitrogen atom also hybridizes in the sp 2. The new orbitals produced in this way are known as hybrid orbitals. There are 5 + 2×6 + 1 = 18 electrons, and 4 are used to make the two covalent. — hybridization is the process of combining pure atomic orbitals on an atom of approximately equal energy to form. What Is The Hybridization Of N.
From socratic.org
What hybridization is generally utilized by the central atom in a What Is The Hybridization Of N Two of the sp 3 hybridized orbitals overlap with s. The nitrogen atom also hybridizes in the sp 2. For example, sp 3 hybridization for nitrogen results in. — hybridization is the process of combining pure atomic orbitals on an atom of approximately equal energy to form a new set of orbitals with the same number as the mixing. What Is The Hybridization Of N.
From saylordotorg.github.io
Localized Bonding and Hybrid Atomic Orbitals What Is The Hybridization Of N There are 5 + 2×6 + 1 = 18 electrons, and 4 are used to make the two covalent. the hybridization schemes for nitrogen and oxygen follow the same guidelines as for carbon. — hybridization is the process of combining pure atomic orbitals on an atom of approximately equal energy to form a new set of orbitals with. What Is The Hybridization Of N.
From www.slideserve.com
PPT Hybridization PowerPoint Presentation, free download ID3367440 What Is The Hybridization Of N the nitrogen is sp 3 hybridized which means that it has four sp 3 hybrid orbitals. For example, sp 3 hybridization for nitrogen results in. There are 5 + 2×6 + 1 = 18 electrons, and 4 are used to make the two covalent. Two of the sp 3 hybridized orbitals overlap with s. the hybridization schemes for. What Is The Hybridization Of N.
From www.chegg.com
Solved Label all bonds in SO2. The hybridization of the S What Is The Hybridization Of N There are 5 + 2×6 + 1 = 18 electrons, and 4 are used to make the two covalent. the nitrogen is sp 3 hybridized which means that it has four sp 3 hybrid orbitals. For example, sp 3 hybridization for nitrogen results in. Nitrogen is the central atom: Two of the sp 3 hybridized orbitals overlap with s.. What Is The Hybridization Of N.
From www.vrogue.co
Lecture 13 Hybridization Examples And Mo Diagram Intr vrogue.co What Is The Hybridization Of N The new orbitals produced in this way are known as hybrid orbitals. Nitrogen is the central atom: the hybridization schemes for nitrogen and oxygen follow the same guidelines as for carbon. For example, sp 3 hybridization for nitrogen results in. The nitrogen atom also hybridizes in the sp 2. There are 5 + 2×6 + 1 = 18 electrons,. What Is The Hybridization Of N.
From guidefixciremerinojx.z21.web.core.windows.net
Valence Bond Diagram What Is The Hybridization Of N Two of the sp 3 hybridized orbitals overlap with s. There are 5 + 2×6 + 1 = 18 electrons, and 4 are used to make the two covalent. Nitrogen is the central atom: The new orbitals produced in this way are known as hybrid orbitals. The nitrogen atom also hybridizes in the sp 2. the nitrogen is sp. What Is The Hybridization Of N.
From exoxqcgtx.blob.core.windows.net
Hybridization Of N Atoms In N2H4 at Alisa McGowan blog What Is The Hybridization Of N For example, sp 3 hybridization for nitrogen results in. Two of the sp 3 hybridized orbitals overlap with s. — hybridization is the process of combining pure atomic orbitals on an atom of approximately equal energy to form a new set of orbitals with the same number as the mixing orbitals, the same energy, and the same shape. The. What Is The Hybridization Of N.
From www.youtube.com
Hybridization of Nitrogen sp3 sp2 and sp Hybridization of Nitrogen What Is The Hybridization Of N the nitrogen is sp 3 hybridized which means that it has four sp 3 hybrid orbitals. There are 5 + 2×6 + 1 = 18 electrons, and 4 are used to make the two covalent. the hybridization schemes for nitrogen and oxygen follow the same guidelines as for carbon. The nitrogen atom also hybridizes in the sp 2.. What Is The Hybridization Of N.
From www.chemistrylibrary.org
Types of Hybridization What Is The Hybridization Of N the hybridization schemes for nitrogen and oxygen follow the same guidelines as for carbon. The nitrogen atom also hybridizes in the sp 2. There are 5 + 2×6 + 1 = 18 electrons, and 4 are used to make the two covalent. — hybridization is the process of combining pure atomic orbitals on an atom of approximately equal. What Is The Hybridization Of N.
From www.youtube.com
NO2 Hybridization (Nitrogen Dioxide) YouTube What Is The Hybridization Of N The new orbitals produced in this way are known as hybrid orbitals. — hybridization is the process of combining pure atomic orbitals on an atom of approximately equal energy to form a new set of orbitals with the same number as the mixing orbitals, the same energy, and the same shape. the hybridization schemes for nitrogen and oxygen. What Is The Hybridization Of N.
From www.pinterest.com.mx
Orbital Hybridization "Cheat Sheet"? + Example Chemistry lessons What Is The Hybridization Of N The nitrogen atom also hybridizes in the sp 2. For example, sp 3 hybridization for nitrogen results in. — hybridization is the process of combining pure atomic orbitals on an atom of approximately equal energy to form a new set of orbitals with the same number as the mixing orbitals, the same energy, and the same shape. There are. What Is The Hybridization Of N.
From ar.inspiredpencil.com
Hybridization Orbitals Chart What Is The Hybridization Of N Nitrogen is the central atom: — hybridization is the process of combining pure atomic orbitals on an atom of approximately equal energy to form a new set of orbitals with the same number as the mixing orbitals, the same energy, and the same shape. There are 5 + 2×6 + 1 = 18 electrons, and 4 are used to. What Is The Hybridization Of N.
From byjus.com
1 What is the hybridisation of nitrogen in pyrrole, pyridine, pepridini What Is The Hybridization Of N — hybridization is the process of combining pure atomic orbitals on an atom of approximately equal energy to form a new set of orbitals with the same number as the mixing orbitals, the same energy, and the same shape. The nitrogen atom also hybridizes in the sp 2. Nitrogen is the central atom: For example, sp 3 hybridization for. What Is The Hybridization Of N.
From talkvol.weebly.com
Hybridization of atomic orbitals talkvol What Is The Hybridization Of N The new orbitals produced in this way are known as hybrid orbitals. Nitrogen is the central atom: There are 5 + 2×6 + 1 = 18 electrons, and 4 are used to make the two covalent. the nitrogen is sp 3 hybridized which means that it has four sp 3 hybrid orbitals. The nitrogen atom also hybridizes in the. What Is The Hybridization Of N.
From www.geeksforgeeks.org
Hybridization Definition, Types, Rules, Examples What Is The Hybridization Of N For example, sp 3 hybridization for nitrogen results in. The new orbitals produced in this way are known as hybrid orbitals. — hybridization is the process of combining pure atomic orbitals on an atom of approximately equal energy to form a new set of orbitals with the same number as the mixing orbitals, the same energy, and the same. What Is The Hybridization Of N.
From www.ochempal.org
Hybridization OChemPal What Is The Hybridization Of N the nitrogen is sp 3 hybridized which means that it has four sp 3 hybrid orbitals. Two of the sp 3 hybridized orbitals overlap with s. Nitrogen is the central atom: There are 5 + 2×6 + 1 = 18 electrons, and 4 are used to make the two covalent. The new orbitals produced in this way are known. What Is The Hybridization Of N.
From byjus.com
37. What is hybridisation of central atom in NO2 What Is The Hybridization Of N the nitrogen is sp 3 hybridized which means that it has four sp 3 hybrid orbitals. There are 5 + 2×6 + 1 = 18 electrons, and 4 are used to make the two covalent. the hybridization schemes for nitrogen and oxygen follow the same guidelines as for carbon. The new orbitals produced in this way are known. What Is The Hybridization Of N.
From chemistrytalk.org
Hybridization and Hybrid Orbitals ChemTalk What Is The Hybridization Of N — hybridization is the process of combining pure atomic orbitals on an atom of approximately equal energy to form a new set of orbitals with the same number as the mixing orbitals, the same energy, and the same shape. There are 5 + 2×6 + 1 = 18 electrons, and 4 are used to make the two covalent. Two. What Is The Hybridization Of N.
From www.masterorganicchemistry.com
How To Determine Hybridization A Shortcut Master Organic Chemistry What Is The Hybridization Of N the hybridization schemes for nitrogen and oxygen follow the same guidelines as for carbon. Nitrogen is the central atom: the nitrogen is sp 3 hybridized which means that it has four sp 3 hybrid orbitals. — hybridization is the process of combining pure atomic orbitals on an atom of approximately equal energy to form a new set. What Is The Hybridization Of N.
From www.masterorganicchemistry.com
How To Determine Hybridization A Shortcut Master Organic Chemistry What Is The Hybridization Of N The new orbitals produced in this way are known as hybrid orbitals. — hybridization is the process of combining pure atomic orbitals on an atom of approximately equal energy to form a new set of orbitals with the same number as the mixing orbitals, the same energy, and the same shape. Nitrogen is the central atom: The nitrogen atom. What Is The Hybridization Of N.
From www.youtube.com
Hybridisation of N2 YouTube What Is The Hybridization Of N There are 5 + 2×6 + 1 = 18 electrons, and 4 are used to make the two covalent. Nitrogen is the central atom: The nitrogen atom also hybridizes in the sp 2. the hybridization schemes for nitrogen and oxygen follow the same guidelines as for carbon. — hybridization is the process of combining pure atomic orbitals on. What Is The Hybridization Of N.
From www.wizeprep.com
Hybridization Wize University Chemistry Textbook Wizeprep What Is The Hybridization Of N The nitrogen atom also hybridizes in the sp 2. Nitrogen is the central atom: the nitrogen is sp 3 hybridized which means that it has four sp 3 hybrid orbitals. Two of the sp 3 hybridized orbitals overlap with s. The new orbitals produced in this way are known as hybrid orbitals. There are 5 + 2×6 + 1. What Is The Hybridization Of N.
From byjus.com
What is the structure and hybridization of CH3 What Is The Hybridization Of N the nitrogen is sp 3 hybridized which means that it has four sp 3 hybrid orbitals. Two of the sp 3 hybridized orbitals overlap with s. — hybridization is the process of combining pure atomic orbitals on an atom of approximately equal energy to form a new set of orbitals with the same number as the mixing orbitals,. What Is The Hybridization Of N.
From general.chemistrysteps.com
Hybridization of Atomic Orbitals Chemistry Steps What Is The Hybridization Of N The new orbitals produced in this way are known as hybrid orbitals. For example, sp 3 hybridization for nitrogen results in. There are 5 + 2×6 + 1 = 18 electrons, and 4 are used to make the two covalent. the nitrogen is sp 3 hybridized which means that it has four sp 3 hybrid orbitals. The nitrogen atom. What Is The Hybridization Of N.
From brainly.in
(e) CH3CH=CHCN what are the type of hybridization Brainly.in What Is The Hybridization Of N The new orbitals produced in this way are known as hybrid orbitals. Nitrogen is the central atom: the hybridization schemes for nitrogen and oxygen follow the same guidelines as for carbon. the nitrogen is sp 3 hybridized which means that it has four sp 3 hybrid orbitals. — hybridization is the process of combining pure atomic orbitals. What Is The Hybridization Of N.
From chem.libretexts.org
Hybridization Chemistry LibreTexts What Is The Hybridization Of N the nitrogen is sp 3 hybridized which means that it has four sp 3 hybrid orbitals. The new orbitals produced in this way are known as hybrid orbitals. Two of the sp 3 hybridized orbitals overlap with s. For example, sp 3 hybridization for nitrogen results in. Nitrogen is the central atom: the hybridization schemes for nitrogen and. What Is The Hybridization Of N.
From www.genome.gov
Hybridization What Is The Hybridization Of N the hybridization schemes for nitrogen and oxygen follow the same guidelines as for carbon. Two of the sp 3 hybridized orbitals overlap with s. The nitrogen atom also hybridizes in the sp 2. Nitrogen is the central atom: — hybridization is the process of combining pure atomic orbitals on an atom of approximately equal energy to form a. What Is The Hybridization Of N.
From www.youtube.com
N2O Hybridization Hybrid Orbitals for N2O YouTube What Is The Hybridization Of N The new orbitals produced in this way are known as hybrid orbitals. Two of the sp 3 hybridized orbitals overlap with s. the nitrogen is sp 3 hybridized which means that it has four sp 3 hybrid orbitals. For example, sp 3 hybridization for nitrogen results in. There are 5 + 2×6 + 1 = 18 electrons, and 4. What Is The Hybridization Of N.
From www.pinterest.com
HYBRIDIZATION Teaching chemistry, Chemistry basics, Organic chemistry What Is The Hybridization Of N The new orbitals produced in this way are known as hybrid orbitals. Two of the sp 3 hybridized orbitals overlap with s. There are 5 + 2×6 + 1 = 18 electrons, and 4 are used to make the two covalent. the nitrogen is sp 3 hybridized which means that it has four sp 3 hybrid orbitals. For example,. What Is The Hybridization Of N.
From www.numerade.com
SOLVEDWhat is the hybridization of each of the carbon and oxygen atoms What Is The Hybridization Of N The new orbitals produced in this way are known as hybrid orbitals. the hybridization schemes for nitrogen and oxygen follow the same guidelines as for carbon. There are 5 + 2×6 + 1 = 18 electrons, and 4 are used to make the two covalent. — hybridization is the process of combining pure atomic orbitals on an atom. What Is The Hybridization Of N.