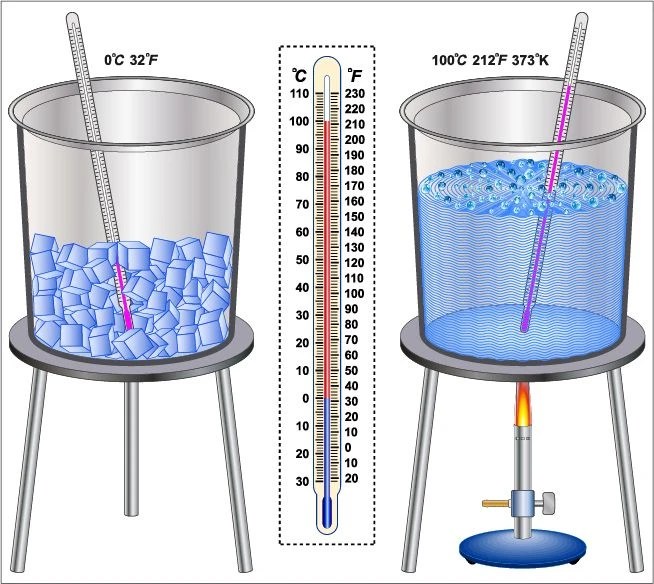
What Is The Boiling Point Of Pure Ice . The melting point of ice is \(0^\text{o} \text{c}\). A common trick to make clear water ice is to boil pure water prior to freezing it. Pure substances have specific melting and boiling points. Mixtures melt and boil over a range of temperatures. Called salol (c 13 h 10 o 3) and. In the deep oceans, under immense pressure, water remains liquid at. At the lower atmospheric pressure on the top of mount everest, pure water boils at about 154 °f (68°c). What is the difference between steam point and boiling point? The melting point of a solid is the same as the freezing point of the liquid. This is the temperature of pure ice melting under standard atmospheric pressure (76cm hg) and is defined. Why does that work and what are the white. The ice point is at 273k and the steam point is at 373k.
from 88guru.com
This is the temperature of pure ice melting under standard atmospheric pressure (76cm hg) and is defined. At the lower atmospheric pressure on the top of mount everest, pure water boils at about 154 °f (68°c). A common trick to make clear water ice is to boil pure water prior to freezing it. What is the difference between steam point and boiling point? In the deep oceans, under immense pressure, water remains liquid at. The ice point is at 273k and the steam point is at 373k. Called salol (c 13 h 10 o 3) and. Mixtures melt and boil over a range of temperatures. Why does that work and what are the white. Pure substances have specific melting and boiling points.
Melting Point of Ice and Boiling Point of Water 88Guru
What Is The Boiling Point Of Pure Ice Mixtures melt and boil over a range of temperatures. This is the temperature of pure ice melting under standard atmospheric pressure (76cm hg) and is defined. Pure substances have specific melting and boiling points. The melting point of a solid is the same as the freezing point of the liquid. Mixtures melt and boil over a range of temperatures. A common trick to make clear water ice is to boil pure water prior to freezing it. At the lower atmospheric pressure on the top of mount everest, pure water boils at about 154 °f (68°c). The melting point of ice is \(0^\text{o} \text{c}\). The ice point is at 273k and the steam point is at 373k. Called salol (c 13 h 10 o 3) and. In the deep oceans, under immense pressure, water remains liquid at. What is the difference between steam point and boiling point? Why does that work and what are the white.
From www.youtube.com
Melting and Boiling Points p98 (Foundation p97) YouTube What Is The Boiling Point Of Pure Ice The melting point of ice is \(0^\text{o} \text{c}\). The melting point of a solid is the same as the freezing point of the liquid. Mixtures melt and boil over a range of temperatures. This is the temperature of pure ice melting under standard atmospheric pressure (76cm hg) and is defined. At the lower atmospheric pressure on the top of mount. What Is The Boiling Point Of Pure Ice.
From www.worksheetsplanet.com
What is Boiling Point What Is The Boiling Point Of Pure Ice At the lower atmospheric pressure on the top of mount everest, pure water boils at about 154 °f (68°c). Pure substances have specific melting and boiling points. The ice point is at 273k and the steam point is at 373k. A common trick to make clear water ice is to boil pure water prior to freezing it. Why does that. What Is The Boiling Point Of Pure Ice.
From 88guru.com
Melting Point of Ice and Boiling Point of Water 88Guru What Is The Boiling Point Of Pure Ice The melting point of ice is \(0^\text{o} \text{c}\). This is the temperature of pure ice melting under standard atmospheric pressure (76cm hg) and is defined. The ice point is at 273k and the steam point is at 373k. Called salol (c 13 h 10 o 3) and. At the lower atmospheric pressure on the top of mount everest, pure water. What Is The Boiling Point Of Pure Ice.
From klaubgmaz.blob.core.windows.net
What Is Boiling Point In Science at Lan Johnson blog What Is The Boiling Point Of Pure Ice Called salol (c 13 h 10 o 3) and. What is the difference between steam point and boiling point? The melting point of a solid is the same as the freezing point of the liquid. At the lower atmospheric pressure on the top of mount everest, pure water boils at about 154 °f (68°c). The melting point of ice is. What Is The Boiling Point Of Pure Ice.
From exyaeabov.blob.core.windows.net
What Is The Boiling Point Melting Point And Freezing Point Of Water at What Is The Boiling Point Of Pure Ice Why does that work and what are the white. Pure substances have specific melting and boiling points. Called salol (c 13 h 10 o 3) and. The melting point of a solid is the same as the freezing point of the liquid. The ice point is at 273k and the steam point is at 373k. In the deep oceans, under. What Is The Boiling Point Of Pure Ice.
From 88guru.com
Melting Point of Ice and Boiling Point of Water 88Guru What Is The Boiling Point Of Pure Ice Called salol (c 13 h 10 o 3) and. A common trick to make clear water ice is to boil pure water prior to freezing it. This is the temperature of pure ice melting under standard atmospheric pressure (76cm hg) and is defined. The melting point of ice is \(0^\text{o} \text{c}\). Mixtures melt and boil over a range of temperatures.. What Is The Boiling Point Of Pure Ice.
From www.youtube.com
COMPARING BOILING POINTS OF PURE SUBSTANCE YouTube What Is The Boiling Point Of Pure Ice A common trick to make clear water ice is to boil pure water prior to freezing it. The melting point of a solid is the same as the freezing point of the liquid. What is the difference between steam point and boiling point? The ice point is at 273k and the steam point is at 373k. Pure substances have specific. What Is The Boiling Point Of Pure Ice.
From ceghryvh.blob.core.windows.net
What Is Boiling Point Sea Level at Edwin Acosta blog What Is The Boiling Point Of Pure Ice In the deep oceans, under immense pressure, water remains liquid at. Called salol (c 13 h 10 o 3) and. What is the difference between steam point and boiling point? The melting point of ice is \(0^\text{o} \text{c}\). A common trick to make clear water ice is to boil pure water prior to freezing it. Pure substances have specific melting. What Is The Boiling Point Of Pure Ice.
From www.savemyexams.com
Melting & Boiling CIE IGCSE Physics Revision Notes 2023 What Is The Boiling Point Of Pure Ice Why does that work and what are the white. This is the temperature of pure ice melting under standard atmospheric pressure (76cm hg) and is defined. The melting point of a solid is the same as the freezing point of the liquid. The melting point of ice is \(0^\text{o} \text{c}\). At the lower atmospheric pressure on the top of mount. What Is The Boiling Point Of Pure Ice.
From studiousguy.com
Boiling Point Examples in Everyday Life StudiousGuy What Is The Boiling Point Of Pure Ice What is the difference between steam point and boiling point? Pure substances have specific melting and boiling points. The melting point of a solid is the same as the freezing point of the liquid. This is the temperature of pure ice melting under standard atmospheric pressure (76cm hg) and is defined. At the lower atmospheric pressure on the top of. What Is The Boiling Point Of Pure Ice.
From mungfali.com
Dry Ice Phase Diagram What Is The Boiling Point Of Pure Ice This is the temperature of pure ice melting under standard atmospheric pressure (76cm hg) and is defined. The melting point of a solid is the same as the freezing point of the liquid. At the lower atmospheric pressure on the top of mount everest, pure water boils at about 154 °f (68°c). What is the difference between steam point and. What Is The Boiling Point Of Pure Ice.
From exyaeabov.blob.core.windows.net
What Is The Boiling Point Melting Point And Freezing Point Of Water at What Is The Boiling Point Of Pure Ice At the lower atmospheric pressure on the top of mount everest, pure water boils at about 154 °f (68°c). Mixtures melt and boil over a range of temperatures. In the deep oceans, under immense pressure, water remains liquid at. This is the temperature of pure ice melting under standard atmospheric pressure (76cm hg) and is defined. Called salol (c 13. What Is The Boiling Point Of Pure Ice.
From fity.club
Melting Points And Boiling Points What Is The Boiling Point Of Pure Ice Pure substances have specific melting and boiling points. This is the temperature of pure ice melting under standard atmospheric pressure (76cm hg) and is defined. In the deep oceans, under immense pressure, water remains liquid at. The melting point of ice is \(0^\text{o} \text{c}\). The melting point of a solid is the same as the freezing point of the liquid.. What Is The Boiling Point Of Pure Ice.
From chemistryskills.com
Definition and Explanation of Boiling Point Chemistry Skills What Is The Boiling Point Of Pure Ice Mixtures melt and boil over a range of temperatures. The melting point of a solid is the same as the freezing point of the liquid. Pure substances have specific melting and boiling points. What is the difference between steam point and boiling point? A common trick to make clear water ice is to boil pure water prior to freezing it.. What Is The Boiling Point Of Pure Ice.
From www.toppr.com
In the Celsius scale, the upper fixed point is What Is The Boiling Point Of Pure Ice Called salol (c 13 h 10 o 3) and. In the deep oceans, under immense pressure, water remains liquid at. The melting point of a solid is the same as the freezing point of the liquid. The melting point of ice is \(0^\text{o} \text{c}\). The ice point is at 273k and the steam point is at 373k. Why does that. What Is The Boiling Point Of Pure Ice.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps What Is The Boiling Point Of Pure Ice The melting point of ice is \(0^\text{o} \text{c}\). A common trick to make clear water ice is to boil pure water prior to freezing it. Pure substances have specific melting and boiling points. At the lower atmospheric pressure on the top of mount everest, pure water boils at about 154 °f (68°c). Mixtures melt and boil over a range of. What Is The Boiling Point Of Pure Ice.
From courses.lumenlearning.com
Colligative Properties Chemistry What Is The Boiling Point Of Pure Ice The ice point is at 273k and the steam point is at 373k. At the lower atmospheric pressure on the top of mount everest, pure water boils at about 154 °f (68°c). The melting point of a solid is the same as the freezing point of the liquid. The melting point of ice is \(0^\text{o} \text{c}\). In the deep oceans,. What Is The Boiling Point Of Pure Ice.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps What Is The Boiling Point Of Pure Ice Mixtures melt and boil over a range of temperatures. Why does that work and what are the white. In the deep oceans, under immense pressure, water remains liquid at. Pure substances have specific melting and boiling points. At the lower atmospheric pressure on the top of mount everest, pure water boils at about 154 °f (68°c). What is the difference. What Is The Boiling Point Of Pure Ice.
From sciencenotes.org
What Is the Freezing Point of Water? Fahrenheit, Celsius, and Kelvin What Is The Boiling Point Of Pure Ice At the lower atmospheric pressure on the top of mount everest, pure water boils at about 154 °f (68°c). Called salol (c 13 h 10 o 3) and. In the deep oceans, under immense pressure, water remains liquid at. The melting point of ice is \(0^\text{o} \text{c}\). What is the difference between steam point and boiling point? The ice point. What Is The Boiling Point Of Pure Ice.
From www.youtube.com
What is Boiling Point Concept of Boiling Point Boiling Point What Is The Boiling Point Of Pure Ice What is the difference between steam point and boiling point? This is the temperature of pure ice melting under standard atmospheric pressure (76cm hg) and is defined. In the deep oceans, under immense pressure, water remains liquid at. At the lower atmospheric pressure on the top of mount everest, pure water boils at about 154 °f (68°c). The melting point. What Is The Boiling Point Of Pure Ice.
From pakmcqs.com
Boiling point of pure water is __________? PakMcqs What Is The Boiling Point Of Pure Ice This is the temperature of pure ice melting under standard atmospheric pressure (76cm hg) and is defined. The melting point of a solid is the same as the freezing point of the liquid. Mixtures melt and boil over a range of temperatures. At the lower atmospheric pressure on the top of mount everest, pure water boils at about 154 °f. What Is The Boiling Point Of Pure Ice.
From exotahhqc.blob.core.windows.net
Water Boils Faster At High Altitudes at Rosa Smith blog What Is The Boiling Point Of Pure Ice The melting point of a solid is the same as the freezing point of the liquid. At the lower atmospheric pressure on the top of mount everest, pure water boils at about 154 °f (68°c). In the deep oceans, under immense pressure, water remains liquid at. This is the temperature of pure ice melting under standard atmospheric pressure (76cm hg). What Is The Boiling Point Of Pure Ice.
From www.youtube.com
Determination of melting point of ice and boiling point of water What Is The Boiling Point Of Pure Ice The melting point of a solid is the same as the freezing point of the liquid. At the lower atmospheric pressure on the top of mount everest, pure water boils at about 154 °f (68°c). In the deep oceans, under immense pressure, water remains liquid at. The melting point of ice is \(0^\text{o} \text{c}\). Pure substances have specific melting and. What Is The Boiling Point Of Pure Ice.
From mavink.com
Melting And Boiling Point Chart What Is The Boiling Point Of Pure Ice Pure substances have specific melting and boiling points. What is the difference between steam point and boiling point? In the deep oceans, under immense pressure, water remains liquid at. At the lower atmospheric pressure on the top of mount everest, pure water boils at about 154 °f (68°c). The melting point of ice is \(0^\text{o} \text{c}\). The ice point is. What Is The Boiling Point Of Pure Ice.
From ar.inspiredpencil.com
Melting Point Of Ice What Is The Boiling Point Of Pure Ice The ice point is at 273k and the steam point is at 373k. What is the difference between steam point and boiling point? In the deep oceans, under immense pressure, water remains liquid at. Mixtures melt and boil over a range of temperatures. The melting point of a solid is the same as the freezing point of the liquid. This. What Is The Boiling Point Of Pure Ice.
From guidemanualeruptivity.z14.web.core.windows.net
Chemistry Boiling Point Chart What Is The Boiling Point Of Pure Ice The melting point of ice is \(0^\text{o} \text{c}\). A common trick to make clear water ice is to boil pure water prior to freezing it. This is the temperature of pure ice melting under standard atmospheric pressure (76cm hg) and is defined. Why does that work and what are the white. Called salol (c 13 h 10 o 3) and.. What Is The Boiling Point Of Pure Ice.
From www.peoi.org
Chapter 10 Section C Properties of Liquids What Is The Boiling Point Of Pure Ice This is the temperature of pure ice melting under standard atmospheric pressure (76cm hg) and is defined. A common trick to make clear water ice is to boil pure water prior to freezing it. The melting point of a solid is the same as the freezing point of the liquid. What is the difference between steam point and boiling point?. What Is The Boiling Point Of Pure Ice.
From www.physicsfox.org
Melting & Boiling • Matter • Physics Fox What Is The Boiling Point Of Pure Ice Why does that work and what are the white. The melting point of a solid is the same as the freezing point of the liquid. What is the difference between steam point and boiling point? This is the temperature of pure ice melting under standard atmospheric pressure (76cm hg) and is defined. Pure substances have specific melting and boiling points.. What Is The Boiling Point Of Pure Ice.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples What Is The Boiling Point Of Pure Ice Called salol (c 13 h 10 o 3) and. Pure substances have specific melting and boiling points. The melting point of ice is \(0^\text{o} \text{c}\). The melting point of a solid is the same as the freezing point of the liquid. Why does that work and what are the white. At the lower atmospheric pressure on the top of mount. What Is The Boiling Point Of Pure Ice.
From fity.club
Melting Points And Boiling Points What Is The Boiling Point Of Pure Ice Called salol (c 13 h 10 o 3) and. Mixtures melt and boil over a range of temperatures. The melting point of ice is \(0^\text{o} \text{c}\). What is the difference between steam point and boiling point? The ice point is at 273k and the steam point is at 373k. This is the temperature of pure ice melting under standard atmospheric. What Is The Boiling Point Of Pure Ice.
From www.vedantu.com
Boiling Point Elevation Learn Important Terms and Concepts What Is The Boiling Point Of Pure Ice What is the difference between steam point and boiling point? This is the temperature of pure ice melting under standard atmospheric pressure (76cm hg) and is defined. At the lower atmospheric pressure on the top of mount everest, pure water boils at about 154 °f (68°c). Pure substances have specific melting and boiling points. In the deep oceans, under immense. What Is The Boiling Point Of Pure Ice.
From stock.adobe.com
Boiling and Evaporation, Freezing and Melting Points of Water. Stock What Is The Boiling Point Of Pure Ice The melting point of ice is \(0^\text{o} \text{c}\). This is the temperature of pure ice melting under standard atmospheric pressure (76cm hg) and is defined. At the lower atmospheric pressure on the top of mount everest, pure water boils at about 154 °f (68°c). Mixtures melt and boil over a range of temperatures. Called salol (c 13 h 10 o. What Is The Boiling Point Of Pure Ice.
From courses.lumenlearning.com
Phase Diagrams Chemistry for Majors What Is The Boiling Point Of Pure Ice In the deep oceans, under immense pressure, water remains liquid at. What is the difference between steam point and boiling point? Mixtures melt and boil over a range of temperatures. The melting point of a solid is the same as the freezing point of the liquid. Why does that work and what are the white. Pure substances have specific melting. What Is The Boiling Point Of Pure Ice.
From www.youtube.com
Science Experiments To Find Out The Boiling Point Of Ice YouTube What Is The Boiling Point Of Pure Ice The melting point of a solid is the same as the freezing point of the liquid. What is the difference between steam point and boiling point? The ice point is at 273k and the steam point is at 373k. In the deep oceans, under immense pressure, water remains liquid at. A common trick to make clear water ice is to. What Is The Boiling Point Of Pure Ice.
From www.gettyimages.com
Freezing And Boiling Points In Celsius And Fahrenheit HighRes Vector What Is The Boiling Point Of Pure Ice The melting point of ice is \(0^\text{o} \text{c}\). At the lower atmospheric pressure on the top of mount everest, pure water boils at about 154 °f (68°c). Called salol (c 13 h 10 o 3) and. In the deep oceans, under immense pressure, water remains liquid at. The melting point of a solid is the same as the freezing point. What Is The Boiling Point Of Pure Ice.