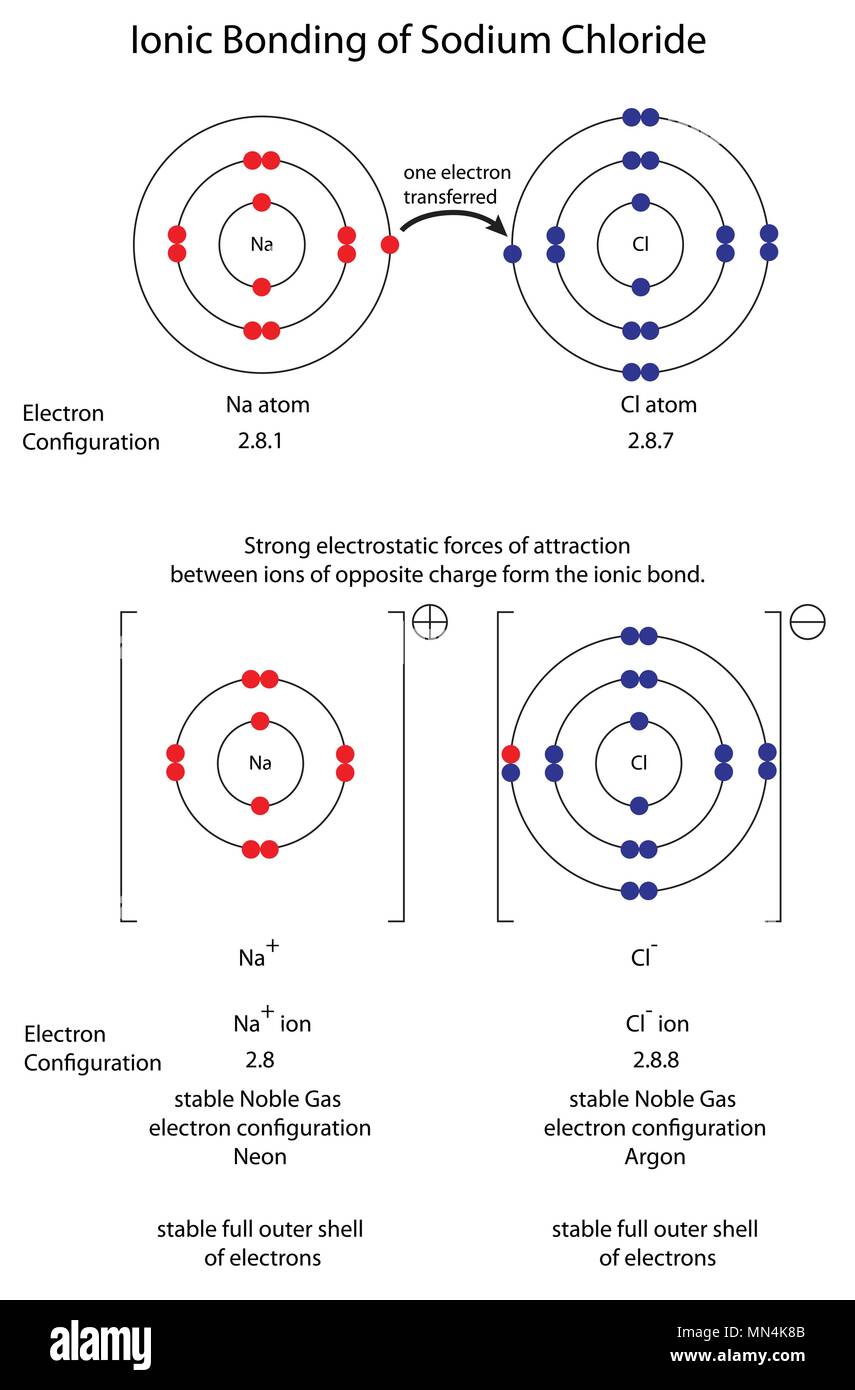
Chlorine Ion Formation . On the right, the chloride ion has 18. The formation of a chloride ion. Similarly, each calcium atom (group 2) can give up two electrons and transfer one to each of two chlorine atoms to form cacl 2, which is. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions. Let’s take a look at how it is formed. On the left, the chlorine atom has 17 electrons. On the left, the chlorine atom has 17 electrons. The formation of a chlorine ion. During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming. Sodium chloride, or table salt, is an ionic compound.
from www.alamy.com
Similarly, each calcium atom (group 2) can give up two electrons and transfer one to each of two chlorine atoms to form cacl 2, which is. On the right, the chloride ion has 18. Let’s take a look at how it is formed. During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming. The formation of a chloride ion. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions. The formation of a chlorine ion. Sodium chloride, or table salt, is an ionic compound. On the left, the chlorine atom has 17 electrons. On the left, the chlorine atom has 17 electrons.
Diagram to show ionic bonding in sodium chloride Stock Vector Image & Art Alamy
Chlorine Ion Formation Similarly, each calcium atom (group 2) can give up two electrons and transfer one to each of two chlorine atoms to form cacl 2, which is. During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming. Sodium chloride, or table salt, is an ionic compound. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions. Similarly, each calcium atom (group 2) can give up two electrons and transfer one to each of two chlorine atoms to form cacl 2, which is. The formation of a chlorine ion. On the left, the chlorine atom has 17 electrons. On the left, the chlorine atom has 17 electrons. The formation of a chloride ion. On the right, the chloride ion has 18. Let’s take a look at how it is formed.
From www.jagranjosh.com
What are Ionic Compounds and how they are formed? Chlorine Ion Formation On the right, the chloride ion has 18. On the left, the chlorine atom has 17 electrons. The formation of a chloride ion. Sodium chloride, or table salt, is an ionic compound. The formation of a chlorine ion. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions. On the left, the. Chlorine Ion Formation.
From basichemistry.blogspot.com
Basic Chemistry Ions, Cations, and Anions Chlorine Ion Formation During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions. Sodium chloride, or table salt, is an ionic compound. On the right, the chloride ion has 18. Similarly, each calcium atom (group 2) can give up two electrons and transfer one to each of two chlorine atoms to form cacl 2, which. Chlorine Ion Formation.
From www.animalia-life.club
Sodium Chloride Lewis Dot Structure Chlorine Ion Formation During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions. Similarly, each calcium atom (group 2) can give up two electrons and transfer one to each of two chlorine atoms to form cacl 2, which is. On the right, the chloride ion has 18. On the left, the chlorine atom has 17. Chlorine Ion Formation.
From www.thesciencehive.co.uk
Ionic Bonding — the science sauce Chlorine Ion Formation Let’s take a look at how it is formed. The formation of a chloride ion. Sodium chloride, or table salt, is an ionic compound. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions. On the right, the chloride ion has 18. During the formation of sodium chloride, the electron given off. Chlorine Ion Formation.
From www.newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Newton Desk Chlorine Ion Formation Let’s take a look at how it is formed. On the right, the chloride ion has 18. The formation of a chlorine ion. On the left, the chlorine atom has 17 electrons. On the left, the chlorine atom has 17 electrons. During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming. The formation. Chlorine Ion Formation.
From www.alamy.com
Diagram to show ionic bonding in sodium chloride Stock Vector Image & Art Alamy Chlorine Ion Formation The formation of a chlorine ion. During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming. On the right, the chloride ion has 18. Similarly, each calcium atom (group 2) can give up two electrons and transfer one to each of two chlorine atoms to form cacl 2, which is. On the left,. Chlorine Ion Formation.
From slidetodoc.com
The Octet Rule Noble Gases the happiest elements Chlorine Ion Formation The formation of a chlorine ion. On the right, the chloride ion has 18. The formation of a chloride ion. Let’s take a look at how it is formed. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions. During the formation of sodium chloride, the electron given off by sodium is. Chlorine Ion Formation.
From surfguppy.com
What is Ionic Bond Surfguppy Chemistry made easy visual learning Chlorine Ion Formation On the left, the chlorine atom has 17 electrons. Similarly, each calcium atom (group 2) can give up two electrons and transfer one to each of two chlorine atoms to form cacl 2, which is. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions. During the formation of sodium chloride, the. Chlorine Ion Formation.
From www2.victoriacollege.edu
formation of ionic bonds Chlorine Ion Formation The formation of a chlorine ion. Similarly, each calcium atom (group 2) can give up two electrons and transfer one to each of two chlorine atoms to form cacl 2, which is. During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming. Sodium chloride, or table salt, is an ionic compound. Let’s take. Chlorine Ion Formation.
From www.essentialchemicalindustry.org
Chlorine Chlorine Ion Formation During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming. The formation of a chlorine ion. The formation of a chloride ion. On the right, the chloride ion has 18. Similarly, each calcium atom (group 2) can give up two electrons and transfer one to each of two chlorine atoms to form cacl. Chlorine Ion Formation.
From www.savemyexams.com
Ions Edexcel GCSE Chemistry Combined Science Revision Notes 2018 Chlorine Ion Formation On the right, the chloride ion has 18. Sodium chloride, or table salt, is an ionic compound. The formation of a chlorine ion. The formation of a chloride ion. On the left, the chlorine atom has 17 electrons. During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming. On the left, the chlorine. Chlorine Ion Formation.
From philschatz.com
Chemical Bonds · Anatomy and Physiology Chlorine Ion Formation The formation of a chlorine ion. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions. On the left, the chlorine atom has 17 electrons. On the left, the chlorine atom has 17 electrons. Sodium chloride, or table salt, is an ionic compound. On the right, the chloride ion has 18. The. Chlorine Ion Formation.
From www.mooramo.com
The Formation of Ionic Compounds From Atoms Mooramo Chlorine Ion Formation On the left, the chlorine atom has 17 electrons. The formation of a chloride ion. Let’s take a look at how it is formed. The formation of a chlorine ion. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions. Similarly, each calcium atom (group 2) can give up two electrons and. Chlorine Ion Formation.
From chem.libretexts.org
4.3 The Reaction of Sodium with Chlorine Chemistry LibreTexts Chlorine Ion Formation On the left, the chlorine atom has 17 electrons. On the right, the chloride ion has 18. Let’s take a look at how it is formed. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions. The formation of a chloride ion. The formation of a chlorine ion. During the formation of. Chlorine Ion Formation.
From tuitiontube.com
Ionic Bond and Ionic Bond Formation, Definition, Properties in Chemistry Tuition Tube Chlorine Ion Formation Let’s take a look at how it is formed. During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming. Sodium chloride, or table salt, is an ionic compound. The formation of a chloride ion. The formation of a chlorine ion. Similarly, each calcium atom (group 2) can give up two electrons and transfer. Chlorine Ion Formation.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica Chlorine Ion Formation Let’s take a look at how it is formed. The formation of a chlorine ion. On the left, the chlorine atom has 17 electrons. During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming. Similarly, each calcium atom (group 2) can give up two electrons and transfer one to each of two chlorine. Chlorine Ion Formation.
From slideplayer.com
Ionic Bonding. ppt download Chlorine Ion Formation The formation of a chlorine ion. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions. The formation of a chloride ion. During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming. Sodium chloride, or table salt, is an ionic compound. Similarly, each calcium atom. Chlorine Ion Formation.
From www.chemistrystudent.com
Ionic Bonding (ALevel) ChemistryStudent Chlorine Ion Formation On the right, the chloride ion has 18. Sodium chloride, or table salt, is an ionic compound. The formation of a chlorine ion. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions. The formation of a chloride ion. On the left, the chlorine atom has 17 electrons. Similarly, each calcium atom. Chlorine Ion Formation.
From www.slideserve.com
PPT Seawater Chemistry PowerPoint Presentation, free download ID1073512 Chlorine Ion Formation Similarly, each calcium atom (group 2) can give up two electrons and transfer one to each of two chlorine atoms to form cacl 2, which is. On the left, the chlorine atom has 17 electrons. Let’s take a look at how it is formed. During the formation of sodium chloride, the electron given off by sodium is taken by chlorine,. Chlorine Ion Formation.
From www.shalom-education.com
Reactions of Halogens GCSE Chemistry Revision Chlorine Ion Formation The formation of a chloride ion. During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions. Let’s take a look at how it is formed. On the left, the chlorine atom has 17 electrons. On. Chlorine Ion Formation.
From www.snexplores.org
Explainer Ions and radicals in our world Chlorine Ion Formation During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming. On the left, the chlorine atom has 17 electrons. On the left, the chlorine atom has 17 electrons. The formation of a chloride ion. On the right, the chloride ion has 18. The formation of a chlorine ion. During the formation of some. Chlorine Ion Formation.
From www.researchgate.net
Formation of chloride ion by ultrasonic degradation of chlorinated... Download Scientific Diagram Chlorine Ion Formation Similarly, each calcium atom (group 2) can give up two electrons and transfer one to each of two chlorine atoms to form cacl 2, which is. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions. During the formation of sodium chloride, the electron given off by sodium is taken by chlorine,. Chlorine Ion Formation.
From byjus.com
Sodium Chloride Preparation, Properties, Structure & Uses Byju's Chlorine Ion Formation On the left, the chlorine atom has 17 electrons. The formation of a chlorine ion. On the left, the chlorine atom has 17 electrons. Let’s take a look at how it is formed. Sodium chloride, or table salt, is an ionic compound. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions.. Chlorine Ion Formation.
From www.shutterstock.com
Vector Illustration Sodium Chloride Formation By Stock Vector (Royalty Free) 2269898333 Chlorine Ion Formation During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions. The formation of a chloride ion. Similarly, each calcium atom (group 2) can give up two electrons and transfer one to each of two chlorine atoms to form cacl 2, which is. Sodium chloride, or table salt, is an ionic compound. During. Chlorine Ion Formation.
From www.dreamstime.com
Ionic Bonding in a Solid Sodium Chloride Crystal Stock Vector Illustration of electron Chlorine Ion Formation Similarly, each calcium atom (group 2) can give up two electrons and transfer one to each of two chlorine atoms to form cacl 2, which is. On the left, the chlorine atom has 17 electrons. The formation of a chloride ion. Let’s take a look at how it is formed. The formation of a chlorine ion. During the formation of. Chlorine Ion Formation.
From www.alamy.com
Sodium Chloride ionic bond formation. NaCl structure. Sodium and Chlorine atom chemical reaction Chlorine Ion Formation During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions. Sodium chloride, or table salt, is an ionic compound. The formation of a chlorine ion. Similarly, each calcium atom (group 2) can give up two electrons and transfer one to each of two chlorine atoms to form cacl 2, which is. On. Chlorine Ion Formation.
From dxohmtoii.blob.core.windows.net
Lewis Dot Structure For Calcium Chlorine at Tony Hammon blog Chlorine Ion Formation The formation of a chlorine ion. Similarly, each calcium atom (group 2) can give up two electrons and transfer one to each of two chlorine atoms to form cacl 2, which is. The formation of a chloride ion. Let’s take a look at how it is formed. On the left, the chlorine atom has 17 electrons. On the left, the. Chlorine Ion Formation.
From slidetodoc.com
Formation of anions negative ions Cl 1 1 Chlorine Ion Formation On the left, the chlorine atom has 17 electrons. On the left, the chlorine atom has 17 electrons. The formation of a chlorine ion. On the right, the chloride ion has 18. During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming. During the formation of some compounds, atoms gain or lose electrons,. Chlorine Ion Formation.
From www.goodscience.com.au
Formation of Ions and Ionic Compounds Good Science Chlorine Ion Formation The formation of a chloride ion. The formation of a chlorine ion. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions. During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming. Similarly, each calcium atom (group 2) can give up two electrons and transfer. Chlorine Ion Formation.
From www.coursehero.com
[Solved] explain the formation and structure of hydrated chloride ions Course Hero Chlorine Ion Formation On the left, the chlorine atom has 17 electrons. Let’s take a look at how it is formed. During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming. On the left, the chlorine atom has 17 electrons. Similarly, each calcium atom (group 2) can give up two electrons and transfer one to each. Chlorine Ion Formation.
From www.expii.com
Ions — Definition & Overview Expii Chlorine Ion Formation Similarly, each calcium atom (group 2) can give up two electrons and transfer one to each of two chlorine atoms to form cacl 2, which is. On the left, the chlorine atom has 17 electrons. On the left, the chlorine atom has 17 electrons. On the right, the chloride ion has 18. Sodium chloride, or table salt, is an ionic. Chlorine Ion Formation.
From www.chemistrystudent.com
Ionic Bonding (ALevel) ChemistryStudent Chlorine Ion Formation Similarly, each calcium atom (group 2) can give up two electrons and transfer one to each of two chlorine atoms to form cacl 2, which is. The formation of a chloride ion. The formation of a chlorine ion. Let’s take a look at how it is formed. During the formation of some compounds, atoms gain or lose electrons, and form. Chlorine Ion Formation.
From www.embibe.com
Draw the atomic structure of the Chlorine atom and chlorine ion Chlorine Ion Formation During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming. On the left, the chlorine atom has 17 electrons. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions. On the right, the chloride ion has 18. Similarly, each calcium atom (group 2) can give. Chlorine Ion Formation.
From slidesharenow.blogspot.com
How Does An Ionic Bond Form Between Sodium And Chlorine slideshare Chlorine Ion Formation On the right, the chloride ion has 18. Similarly, each calcium atom (group 2) can give up two electrons and transfer one to each of two chlorine atoms to form cacl 2, which is. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions. On the left, the chlorine atom has 17. Chlorine Ion Formation.
From www.bartleby.com
B. A chlorine atom (Cl) a negatively charged chloride ion (Cl − ) when it gains an Chlorine Ion Formation Similarly, each calcium atom (group 2) can give up two electrons and transfer one to each of two chlorine atoms to form cacl 2, which is. During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called. Chlorine Ion Formation.