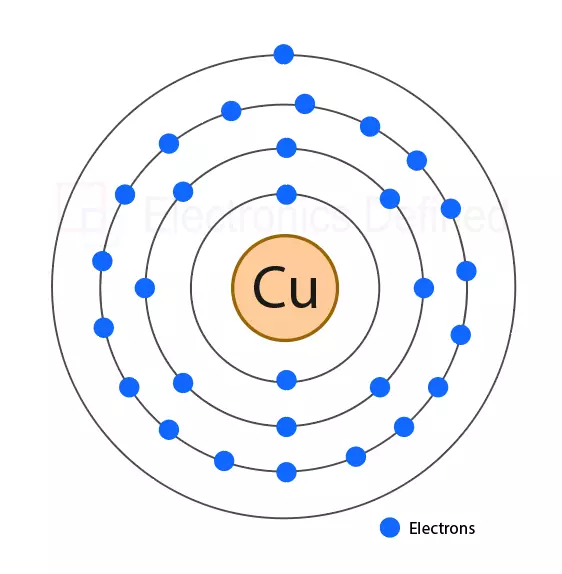
Does Copper Conduct Electricity Better Than Aluminum . However, when looking at lifetime costs, there remains no economic advantage to using the. Another factor that influences a metal's conductivity is its purity, since impurities interfere with the movement of free electrons through the solid. By the same token, the most effective conductors of electricity are metals that have a single valence electron that is free to move and causes a strong. Even though copper has a long history as the material of choice for conducting electricity, aluminum has certain advantages that make it. For example, pure copper (cu) is more conductive than a copper alloy —a substance combining copper and one or more other chemical elements, each of which is essentially an impurity. It is a durable and reliable conductor, especially for applications where performance. Copper conductors come at a premium price. Aluminum conducts electricity 61 percent as well as copper but weighs 1/3 as much. Generally speaking, aluminium cable will be substantially cheaper. Because of this, aluminum conductors are less expensive than conductors made of copper. However, it is still worth recalling that copper cable is more ductile and less susceptible to electrical contact. Aluminium is abundantly available and offers a cheaper alternative to copper for conductors. On the other hand, copper excels in terms of electrical conductivity, corrosion resistance, and ampacity. The demand for copper is variable and the price.
from electronicsdefined.com
However, when looking at lifetime costs, there remains no economic advantage to using the. Another factor that influences a metal's conductivity is its purity, since impurities interfere with the movement of free electrons through the solid. On the other hand, copper excels in terms of electrical conductivity, corrosion resistance, and ampacity. By the same token, the most effective conductors of electricity are metals that have a single valence electron that is free to move and causes a strong. Copper conductors come at a premium price. Generally speaking, aluminium cable will be substantially cheaper. Because of this, aluminum conductors are less expensive than conductors made of copper. Even though copper has a long history as the material of choice for conducting electricity, aluminum has certain advantages that make it. However, it is still worth recalling that copper cable is more ductile and less susceptible to electrical contact. Aluminum conducts electricity 61 percent as well as copper but weighs 1/3 as much.
Why do metals conduct electricity? Learn science behind the electrical
Does Copper Conduct Electricity Better Than Aluminum The demand for copper is variable and the price. Because of this, aluminum conductors are less expensive than conductors made of copper. Even though copper has a long history as the material of choice for conducting electricity, aluminum has certain advantages that make it. Another factor that influences a metal's conductivity is its purity, since impurities interfere with the movement of free electrons through the solid. On the other hand, copper excels in terms of electrical conductivity, corrosion resistance, and ampacity. Copper conductors come at a premium price. By the same token, the most effective conductors of electricity are metals that have a single valence electron that is free to move and causes a strong. Generally speaking, aluminium cable will be substantially cheaper. Aluminum conducts electricity 61 percent as well as copper but weighs 1/3 as much. However, when looking at lifetime costs, there remains no economic advantage to using the. However, it is still worth recalling that copper cable is more ductile and less susceptible to electrical contact. It is a durable and reliable conductor, especially for applications where performance. The demand for copper is variable and the price. Aluminium is abundantly available and offers a cheaper alternative to copper for conductors. For example, pure copper (cu) is more conductive than a copper alloy —a substance combining copper and one or more other chemical elements, each of which is essentially an impurity.
From www.thoughtco.com
Electrical Conductivity of Metals Does Copper Conduct Electricity Better Than Aluminum Aluminum conducts electricity 61 percent as well as copper but weighs 1/3 as much. Copper conductors come at a premium price. The demand for copper is variable and the price. Aluminium is abundantly available and offers a cheaper alternative to copper for conductors. It is a durable and reliable conductor, especially for applications where performance. Another factor that influences a. Does Copper Conduct Electricity Better Than Aluminum.
From blog.thepipingmart.com
Benefits of Using Copper for Electrical Conductivity and Heat Resistance Does Copper Conduct Electricity Better Than Aluminum Aluminium is abundantly available and offers a cheaper alternative to copper for conductors. By the same token, the most effective conductors of electricity are metals that have a single valence electron that is free to move and causes a strong. For example, pure copper (cu) is more conductive than a copper alloy —a substance combining copper and one or more. Does Copper Conduct Electricity Better Than Aluminum.
From techiescientist.com
Does Copper Conduct Electricity? Techiescientist Does Copper Conduct Electricity Better Than Aluminum Because of this, aluminum conductors are less expensive than conductors made of copper. Generally speaking, aluminium cable will be substantially cheaper. However, it is still worth recalling that copper cable is more ductile and less susceptible to electrical contact. Aluminum conducts electricity 61 percent as well as copper but weighs 1/3 as much. By the same token, the most effective. Does Copper Conduct Electricity Better Than Aluminum.
From dmaengineers.com.au
Copper vs Aluminium cables which is best for electrical installations Does Copper Conduct Electricity Better Than Aluminum Copper conductors come at a premium price. Another factor that influences a metal's conductivity is its purity, since impurities interfere with the movement of free electrons through the solid. Even though copper has a long history as the material of choice for conducting electricity, aluminum has certain advantages that make it. However, when looking at lifetime costs, there remains no. Does Copper Conduct Electricity Better Than Aluminum.
From study.com
Conductivity of Aluminum Overview & Properties Lesson Does Copper Conduct Electricity Better Than Aluminum Generally speaking, aluminium cable will be substantially cheaper. For example, pure copper (cu) is more conductive than a copper alloy —a substance combining copper and one or more other chemical elements, each of which is essentially an impurity. However, it is still worth recalling that copper cable is more ductile and less susceptible to electrical contact. Copper conductors come at. Does Copper Conduct Electricity Better Than Aluminum.
From blog.thepipingmart.com
Aluminium vs Copper Wire What's the Difference Does Copper Conduct Electricity Better Than Aluminum Even though copper has a long history as the material of choice for conducting electricity, aluminum has certain advantages that make it. Copper conductors come at a premium price. On the other hand, copper excels in terms of electrical conductivity, corrosion resistance, and ampacity. Because of this, aluminum conductors are less expensive than conductors made of copper. Aluminium is abundantly. Does Copper Conduct Electricity Better Than Aluminum.
From www.slideserve.com
PPT AQA GCSE 1a1 Heat Transfer PowerPoint Presentation, free Does Copper Conduct Electricity Better Than Aluminum It is a durable and reliable conductor, especially for applications where performance. For example, pure copper (cu) is more conductive than a copper alloy —a substance combining copper and one or more other chemical elements, each of which is essentially an impurity. On the other hand, copper excels in terms of electrical conductivity, corrosion resistance, and ampacity. Another factor that. Does Copper Conduct Electricity Better Than Aluminum.
From www.slideshare.net
Chapter 24 Conduction Does Copper Conduct Electricity Better Than Aluminum Because of this, aluminum conductors are less expensive than conductors made of copper. Another factor that influences a metal's conductivity is its purity, since impurities interfere with the movement of free electrons through the solid. The demand for copper is variable and the price. It is a durable and reliable conductor, especially for applications where performance. However, it is still. Does Copper Conduct Electricity Better Than Aluminum.
From enginelibraryangela.z19.web.core.windows.net
Copper Vs Aluminum Wiring Does Copper Conduct Electricity Better Than Aluminum Even though copper has a long history as the material of choice for conducting electricity, aluminum has certain advantages that make it. However, when looking at lifetime costs, there remains no economic advantage to using the. Generally speaking, aluminium cable will be substantially cheaper. However, it is still worth recalling that copper cable is more ductile and less susceptible to. Does Copper Conduct Electricity Better Than Aluminum.
From techiescientist.com
Does Copper Conduct Electricity? Techiescientist Does Copper Conduct Electricity Better Than Aluminum Because of this, aluminum conductors are less expensive than conductors made of copper. Aluminum conducts electricity 61 percent as well as copper but weighs 1/3 as much. Even though copper has a long history as the material of choice for conducting electricity, aluminum has certain advantages that make it. Aluminium is abundantly available and offers a cheaper alternative to copper. Does Copper Conduct Electricity Better Than Aluminum.
From www.youtube.com
Do different metals conduct electricity equally well? YouTube Does Copper Conduct Electricity Better Than Aluminum Generally speaking, aluminium cable will be substantially cheaper. Aluminium is abundantly available and offers a cheaper alternative to copper for conductors. However, it is still worth recalling that copper cable is more ductile and less susceptible to electrical contact. The demand for copper is variable and the price. Because of this, aluminum conductors are less expensive than conductors made of. Does Copper Conduct Electricity Better Than Aluminum.
From www.electricaldesks.com
If same power is transmitted on copper conductor line and on aluminium Does Copper Conduct Electricity Better Than Aluminum Because of this, aluminum conductors are less expensive than conductors made of copper. By the same token, the most effective conductors of electricity are metals that have a single valence electron that is free to move and causes a strong. Aluminium is abundantly available and offers a cheaper alternative to copper for conductors. However, when looking at lifetime costs, there. Does Copper Conduct Electricity Better Than Aluminum.
From learningdbpfeifer.z21.web.core.windows.net
Electrical Conductivity Of Metals Table Does Copper Conduct Electricity Better Than Aluminum By the same token, the most effective conductors of electricity are metals that have a single valence electron that is free to move and causes a strong. However, it is still worth recalling that copper cable is more ductile and less susceptible to electrical contact. It is a durable and reliable conductor, especially for applications where performance. Aluminum conducts electricity. Does Copper Conduct Electricity Better Than Aluminum.
From diagramlistregina.z6.web.core.windows.net
Aluminum Vs Copper Wire Conductivity Does Copper Conduct Electricity Better Than Aluminum It is a durable and reliable conductor, especially for applications where performance. Another factor that influences a metal's conductivity is its purity, since impurities interfere with the movement of free electrons through the solid. Generally speaking, aluminium cable will be substantially cheaper. Even though copper has a long history as the material of choice for conducting electricity, aluminum has certain. Does Copper Conduct Electricity Better Than Aluminum.
From www.greentechrenewables.com
Aluminum Vs. Copper Greentech Renewables Does Copper Conduct Electricity Better Than Aluminum Another factor that influences a metal's conductivity is its purity, since impurities interfere with the movement of free electrons through the solid. It is a durable and reliable conductor, especially for applications where performance. Copper conductors come at a premium price. The demand for copper is variable and the price. On the other hand, copper excels in terms of electrical. Does Copper Conduct Electricity Better Than Aluminum.
From www.youtube.com
Why do metals conduct electricity better than other? YouTube Does Copper Conduct Electricity Better Than Aluminum On the other hand, copper excels in terms of electrical conductivity, corrosion resistance, and ampacity. However, it is still worth recalling that copper cable is more ductile and less susceptible to electrical contact. Copper conductors come at a premium price. Another factor that influences a metal's conductivity is its purity, since impurities interfere with the movement of free electrons through. Does Copper Conduct Electricity Better Than Aluminum.
From telgurus.co.uk
How do metals conduct electricity? Chemistry Questions TEL Gurus Does Copper Conduct Electricity Better Than Aluminum However, when looking at lifetime costs, there remains no economic advantage to using the. Aluminium is abundantly available and offers a cheaper alternative to copper for conductors. Another factor that influences a metal's conductivity is its purity, since impurities interfere with the movement of free electrons through the solid. Generally speaking, aluminium cable will be substantially cheaper. Even though copper. Does Copper Conduct Electricity Better Than Aluminum.
From www.slideshare.net
5.1 Potential Difference, Current & Resistance Does Copper Conduct Electricity Better Than Aluminum Copper conductors come at a premium price. Even though copper has a long history as the material of choice for conducting electricity, aluminum has certain advantages that make it. On the other hand, copper excels in terms of electrical conductivity, corrosion resistance, and ampacity. For example, pure copper (cu) is more conductive than a copper alloy —a substance combining copper. Does Copper Conduct Electricity Better Than Aluminum.
From www.youtube.com
Electrical Connectability Copper versus Aluminum YouTube Does Copper Conduct Electricity Better Than Aluminum Copper conductors come at a premium price. The demand for copper is variable and the price. Because of this, aluminum conductors are less expensive than conductors made of copper. Aluminum conducts electricity 61 percent as well as copper but weighs 1/3 as much. By the same token, the most effective conductors of electricity are metals that have a single valence. Does Copper Conduct Electricity Better Than Aluminum.
From www.youtube.com
Aluminum v. Copper Wiring YouTube Does Copper Conduct Electricity Better Than Aluminum On the other hand, copper excels in terms of electrical conductivity, corrosion resistance, and ampacity. Aluminium is abundantly available and offers a cheaper alternative to copper for conductors. For example, pure copper (cu) is more conductive than a copper alloy —a substance combining copper and one or more other chemical elements, each of which is essentially an impurity. By the. Does Copper Conduct Electricity Better Than Aluminum.
From www.slideshare.net
Chapter 24 Conduction Does Copper Conduct Electricity Better Than Aluminum Because of this, aluminum conductors are less expensive than conductors made of copper. However, when looking at lifetime costs, there remains no economic advantage to using the. It is a durable and reliable conductor, especially for applications where performance. Another factor that influences a metal's conductivity is its purity, since impurities interfere with the movement of free electrons through the. Does Copper Conduct Electricity Better Than Aluminum.
From www.windsystemsmag.com
Aluminum Vs. Copper Conductors Wind Systems Magazine Does Copper Conduct Electricity Better Than Aluminum Aluminium is abundantly available and offers a cheaper alternative to copper for conductors. On the other hand, copper excels in terms of electrical conductivity, corrosion resistance, and ampacity. However, it is still worth recalling that copper cable is more ductile and less susceptible to electrical contact. It is a durable and reliable conductor, especially for applications where performance. Even though. Does Copper Conduct Electricity Better Than Aluminum.
From www.teachoo.com
NCERT Q18 (e) Why are copper and aluminium wires usually employed Does Copper Conduct Electricity Better Than Aluminum However, it is still worth recalling that copper cable is more ductile and less susceptible to electrical contact. Copper conductors come at a premium price. Because of this, aluminum conductors are less expensive than conductors made of copper. Generally speaking, aluminium cable will be substantially cheaper. Another factor that influences a metal's conductivity is its purity, since impurities interfere with. Does Copper Conduct Electricity Better Than Aluminum.
From joiruwium.blob.core.windows.net
Copper Is A Good Conductor Of Electricity And Heat Justify The Does Copper Conduct Electricity Better Than Aluminum Aluminium is abundantly available and offers a cheaper alternative to copper for conductors. It is a durable and reliable conductor, especially for applications where performance. For example, pure copper (cu) is more conductive than a copper alloy —a substance combining copper and one or more other chemical elements, each of which is essentially an impurity. On the other hand, copper. Does Copper Conduct Electricity Better Than Aluminum.
From www.sequoia-brass-copper.com
Which Metals Conduct Electricity? Does Copper Conduct Electricity Better Than Aluminum However, when looking at lifetime costs, there remains no economic advantage to using the. Aluminium is abundantly available and offers a cheaper alternative to copper for conductors. For example, pure copper (cu) is more conductive than a copper alloy —a substance combining copper and one or more other chemical elements, each of which is essentially an impurity. Because of this,. Does Copper Conduct Electricity Better Than Aluminum.
From joikjmxpr.blob.core.windows.net
Name The Metal Which Is The Best Conductor Of Heat And Electricity at Does Copper Conduct Electricity Better Than Aluminum Another factor that influences a metal's conductivity is its purity, since impurities interfere with the movement of free electrons through the solid. For example, pure copper (cu) is more conductive than a copper alloy —a substance combining copper and one or more other chemical elements, each of which is essentially an impurity. However, it is still worth recalling that copper. Does Copper Conduct Electricity Better Than Aluminum.
From electronicsdefined.com
Why do metals conduct electricity? Learn science behind the electrical Does Copper Conduct Electricity Better Than Aluminum On the other hand, copper excels in terms of electrical conductivity, corrosion resistance, and ampacity. For example, pure copper (cu) is more conductive than a copper alloy —a substance combining copper and one or more other chemical elements, each of which is essentially an impurity. The demand for copper is variable and the price. Another factor that influences a metal's. Does Copper Conduct Electricity Better Than Aluminum.
From msestudent.com
Why Do Metals Conduct Electricity? Materials Science & Engineering Does Copper Conduct Electricity Better Than Aluminum It is a durable and reliable conductor, especially for applications where performance. The demand for copper is variable and the price. However, it is still worth recalling that copper cable is more ductile and less susceptible to electrical contact. Because of this, aluminum conductors are less expensive than conductors made of copper. Aluminum conducts electricity 61 percent as well as. Does Copper Conduct Electricity Better Than Aluminum.
From msestudent.com
Why Do Metals Conduct Electricity? Materials Science & Engineering Does Copper Conduct Electricity Better Than Aluminum For example, pure copper (cu) is more conductive than a copper alloy —a substance combining copper and one or more other chemical elements, each of which is essentially an impurity. However, when looking at lifetime costs, there remains no economic advantage to using the. Even though copper has a long history as the material of choice for conducting electricity, aluminum. Does Copper Conduct Electricity Better Than Aluminum.
From www.thoughtco.com
10 Examples of Electrical Conductors and Insulators Does Copper Conduct Electricity Better Than Aluminum Copper conductors come at a premium price. The demand for copper is variable and the price. Another factor that influences a metal's conductivity is its purity, since impurities interfere with the movement of free electrons through the solid. However, it is still worth recalling that copper cable is more ductile and less susceptible to electrical contact. Aluminum conducts electricity 61. Does Copper Conduct Electricity Better Than Aluminum.
From www.assignments4u.com
Why do Metals Conduct Electricity Detailed Guide Does Copper Conduct Electricity Better Than Aluminum On the other hand, copper excels in terms of electrical conductivity, corrosion resistance, and ampacity. By the same token, the most effective conductors of electricity are metals that have a single valence electron that is free to move and causes a strong. Because of this, aluminum conductors are less expensive than conductors made of copper. Aluminium is abundantly available and. Does Copper Conduct Electricity Better Than Aluminum.
From wiringdbuta101.z19.web.core.windows.net
Aluminum Wiring Vs Copper Does Copper Conduct Electricity Better Than Aluminum Another factor that influences a metal's conductivity is its purity, since impurities interfere with the movement of free electrons through the solid. For example, pure copper (cu) is more conductive than a copper alloy —a substance combining copper and one or more other chemical elements, each of which is essentially an impurity. Aluminium is abundantly available and offers a cheaper. Does Copper Conduct Electricity Better Than Aluminum.
From www.groupnirmal.com
Copper Wire V/S Aluminum Wire Group Nirmal Does Copper Conduct Electricity Better Than Aluminum Aluminum conducts electricity 61 percent as well as copper but weighs 1/3 as much. Generally speaking, aluminium cable will be substantially cheaper. Even though copper has a long history as the material of choice for conducting electricity, aluminum has certain advantages that make it. It is a durable and reliable conductor, especially for applications where performance. Because of this, aluminum. Does Copper Conduct Electricity Better Than Aluminum.
From gabrielcoates.z13.web.core.windows.net
Electrical Conductivity Of Metals Chart Does Copper Conduct Electricity Better Than Aluminum Because of this, aluminum conductors are less expensive than conductors made of copper. Aluminium is abundantly available and offers a cheaper alternative to copper for conductors. It is a durable and reliable conductor, especially for applications where performance. Copper conductors come at a premium price. Even though copper has a long history as the material of choice for conducting electricity,. Does Copper Conduct Electricity Better Than Aluminum.
From www.electricity-magnetism.org
How do metals conduct electricity? Does Copper Conduct Electricity Better Than Aluminum It is a durable and reliable conductor, especially for applications where performance. Because of this, aluminum conductors are less expensive than conductors made of copper. By the same token, the most effective conductors of electricity are metals that have a single valence electron that is free to move and causes a strong. Even though copper has a long history as. Does Copper Conduct Electricity Better Than Aluminum.