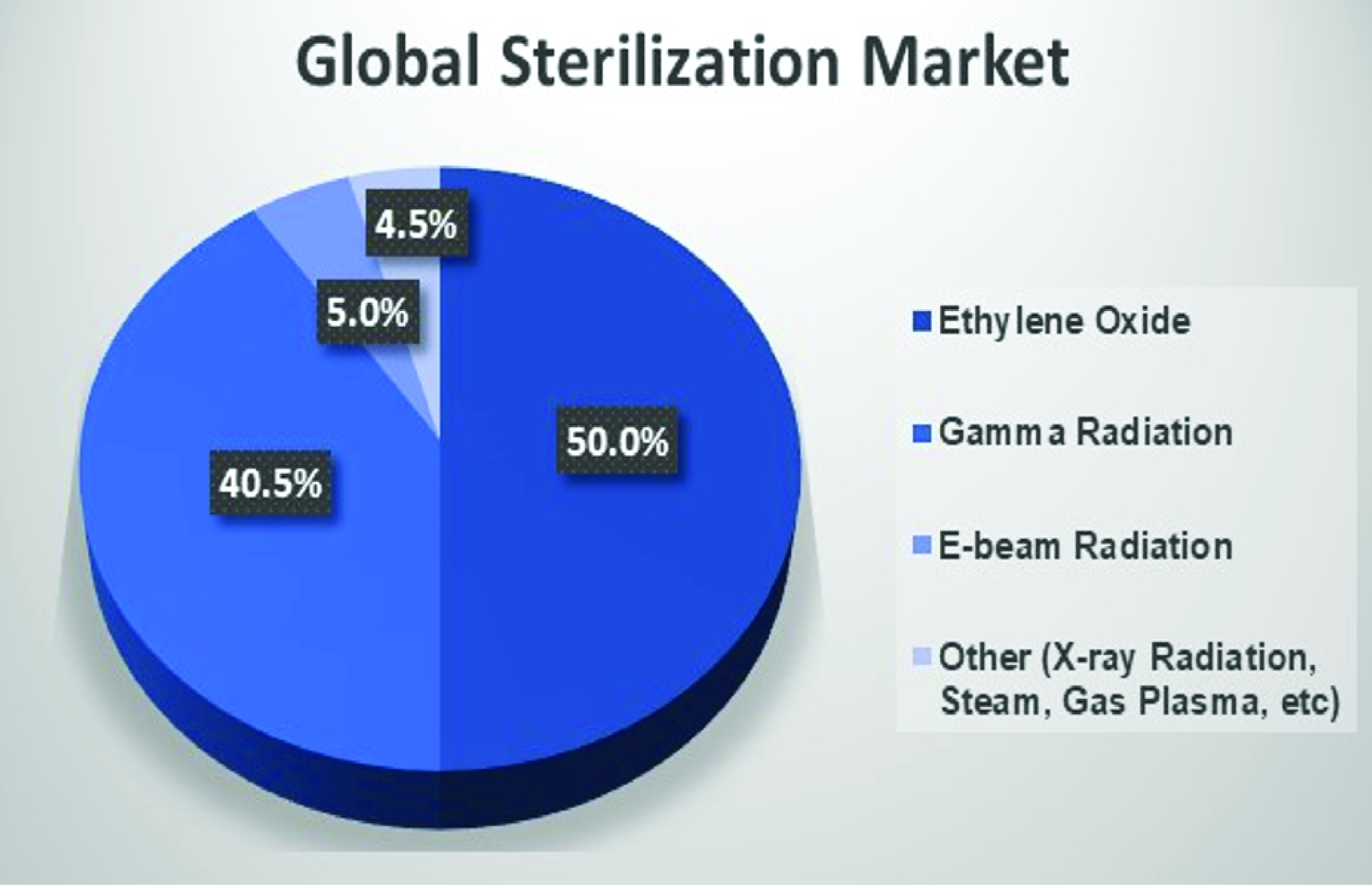
Radiation Sterilization Of Medical Devices . On june 7, 2022, the fda announced it was considering a master file pilot program for premarket approval (pma) holders whose. A list of all parts in the iso 11137. This part of iso 11137 describes requirements that, if met, will provide a radiation sterilization process intended to sterilize medical devices, that has. This document was prepared by technical committee iso/tc 198, sterilization of health care products. The food and drug administration (fda) requires sterilization of all invasive medical and dental devices including syringes, surgical. Radiation is a clean method of sterilization, as it does not require any chemical treatment and therefore does not leave any residues behind.
from large.stanford.edu
This part of iso 11137 describes requirements that, if met, will provide a radiation sterilization process intended to sterilize medical devices, that has. Radiation is a clean method of sterilization, as it does not require any chemical treatment and therefore does not leave any residues behind. The food and drug administration (fda) requires sterilization of all invasive medical and dental devices including syringes, surgical. A list of all parts in the iso 11137. This document was prepared by technical committee iso/tc 198, sterilization of health care products. On june 7, 2022, the fda announced it was considering a master file pilot program for premarket approval (pma) holders whose.
Radiation Sterilization
Radiation Sterilization Of Medical Devices This part of iso 11137 describes requirements that, if met, will provide a radiation sterilization process intended to sterilize medical devices, that has. This document was prepared by technical committee iso/tc 198, sterilization of health care products. A list of all parts in the iso 11137. This part of iso 11137 describes requirements that, if met, will provide a radiation sterilization process intended to sterilize medical devices, that has. Radiation is a clean method of sterilization, as it does not require any chemical treatment and therefore does not leave any residues behind. On june 7, 2022, the fda announced it was considering a master file pilot program for premarket approval (pma) holders whose. The food and drug administration (fda) requires sterilization of all invasive medical and dental devices including syringes, surgical.
From
Radiation Sterilization Of Medical Devices This document was prepared by technical committee iso/tc 198, sterilization of health care products. This part of iso 11137 describes requirements that, if met, will provide a radiation sterilization process intended to sterilize medical devices, that has. A list of all parts in the iso 11137. On june 7, 2022, the fda announced it was considering a master file pilot. Radiation Sterilization Of Medical Devices.
From
Radiation Sterilization Of Medical Devices On june 7, 2022, the fda announced it was considering a master file pilot program for premarket approval (pma) holders whose. A list of all parts in the iso 11137. This document was prepared by technical committee iso/tc 198, sterilization of health care products. This part of iso 11137 describes requirements that, if met, will provide a radiation sterilization process. Radiation Sterilization Of Medical Devices.
From
Radiation Sterilization Of Medical Devices A list of all parts in the iso 11137. Radiation is a clean method of sterilization, as it does not require any chemical treatment and therefore does not leave any residues behind. This part of iso 11137 describes requirements that, if met, will provide a radiation sterilization process intended to sterilize medical devices, that has. This document was prepared by. Radiation Sterilization Of Medical Devices.
From
Radiation Sterilization Of Medical Devices Radiation is a clean method of sterilization, as it does not require any chemical treatment and therefore does not leave any residues behind. On june 7, 2022, the fda announced it was considering a master file pilot program for premarket approval (pma) holders whose. This document was prepared by technical committee iso/tc 198, sterilization of health care products. This part. Radiation Sterilization Of Medical Devices.
From
Radiation Sterilization Of Medical Devices On june 7, 2022, the fda announced it was considering a master file pilot program for premarket approval (pma) holders whose. The food and drug administration (fda) requires sterilization of all invasive medical and dental devices including syringes, surgical. A list of all parts in the iso 11137. This part of iso 11137 describes requirements that, if met, will provide. Radiation Sterilization Of Medical Devices.
From
Radiation Sterilization Of Medical Devices The food and drug administration (fda) requires sterilization of all invasive medical and dental devices including syringes, surgical. Radiation is a clean method of sterilization, as it does not require any chemical treatment and therefore does not leave any residues behind. This part of iso 11137 describes requirements that, if met, will provide a radiation sterilization process intended to sterilize. Radiation Sterilization Of Medical Devices.
From
Radiation Sterilization Of Medical Devices This part of iso 11137 describes requirements that, if met, will provide a radiation sterilization process intended to sterilize medical devices, that has. This document was prepared by technical committee iso/tc 198, sterilization of health care products. A list of all parts in the iso 11137. Radiation is a clean method of sterilization, as it does not require any chemical. Radiation Sterilization Of Medical Devices.
From
Radiation Sterilization Of Medical Devices Radiation is a clean method of sterilization, as it does not require any chemical treatment and therefore does not leave any residues behind. On june 7, 2022, the fda announced it was considering a master file pilot program for premarket approval (pma) holders whose. A list of all parts in the iso 11137. This document was prepared by technical committee. Radiation Sterilization Of Medical Devices.
From www.aami.org
Radiation Sterilization for Medical Devices AAMI Radiation Sterilization Of Medical Devices This document was prepared by technical committee iso/tc 198, sterilization of health care products. The food and drug administration (fda) requires sterilization of all invasive medical and dental devices including syringes, surgical. A list of all parts in the iso 11137. Radiation is a clean method of sterilization, as it does not require any chemical treatment and therefore does not. Radiation Sterilization Of Medical Devices.
From
Radiation Sterilization Of Medical Devices On june 7, 2022, the fda announced it was considering a master file pilot program for premarket approval (pma) holders whose. This part of iso 11137 describes requirements that, if met, will provide a radiation sterilization process intended to sterilize medical devices, that has. Radiation is a clean method of sterilization, as it does not require any chemical treatment and. Radiation Sterilization Of Medical Devices.
From
Radiation Sterilization Of Medical Devices This part of iso 11137 describes requirements that, if met, will provide a radiation sterilization process intended to sterilize medical devices, that has. The food and drug administration (fda) requires sterilization of all invasive medical and dental devices including syringes, surgical. Radiation is a clean method of sterilization, as it does not require any chemical treatment and therefore does not. Radiation Sterilization Of Medical Devices.
From
Radiation Sterilization Of Medical Devices Radiation is a clean method of sterilization, as it does not require any chemical treatment and therefore does not leave any residues behind. A list of all parts in the iso 11137. This part of iso 11137 describes requirements that, if met, will provide a radiation sterilization process intended to sterilize medical devices, that has. This document was prepared by. Radiation Sterilization Of Medical Devices.
From
Radiation Sterilization Of Medical Devices The food and drug administration (fda) requires sterilization of all invasive medical and dental devices including syringes, surgical. A list of all parts in the iso 11137. This part of iso 11137 describes requirements that, if met, will provide a radiation sterilization process intended to sterilize medical devices, that has. Radiation is a clean method of sterilization, as it does. Radiation Sterilization Of Medical Devices.
From 24x7mag.com
FDA Considers Pilot Program for Radiation Sterilization of Medical Radiation Sterilization Of Medical Devices This document was prepared by technical committee iso/tc 198, sterilization of health care products. Radiation is a clean method of sterilization, as it does not require any chemical treatment and therefore does not leave any residues behind. On june 7, 2022, the fda announced it was considering a master file pilot program for premarket approval (pma) holders whose. This part. Radiation Sterilization Of Medical Devices.
From www.researchgate.net
(PDF) Trends in Radiation Sterilization of Health Care Products Radiation Sterilization Of Medical Devices The food and drug administration (fda) requires sterilization of all invasive medical and dental devices including syringes, surgical. This document was prepared by technical committee iso/tc 198, sterilization of health care products. Radiation is a clean method of sterilization, as it does not require any chemical treatment and therefore does not leave any residues behind. On june 7, 2022, the. Radiation Sterilization Of Medical Devices.
From
Radiation Sterilization Of Medical Devices Radiation is a clean method of sterilization, as it does not require any chemical treatment and therefore does not leave any residues behind. A list of all parts in the iso 11137. The food and drug administration (fda) requires sterilization of all invasive medical and dental devices including syringes, surgical. This document was prepared by technical committee iso/tc 198, sterilization. Radiation Sterilization Of Medical Devices.
From
Radiation Sterilization Of Medical Devices This document was prepared by technical committee iso/tc 198, sterilization of health care products. A list of all parts in the iso 11137. The food and drug administration (fda) requires sterilization of all invasive medical and dental devices including syringes, surgical. On june 7, 2022, the fda announced it was considering a master file pilot program for premarket approval (pma). Radiation Sterilization Of Medical Devices.
From www.scientistlive.com
Radiation sterilisation firm expands Scientist Live Radiation Sterilization Of Medical Devices This part of iso 11137 describes requirements that, if met, will provide a radiation sterilization process intended to sterilize medical devices, that has. The food and drug administration (fda) requires sterilization of all invasive medical and dental devices including syringes, surgical. Radiation is a clean method of sterilization, as it does not require any chemical treatment and therefore does not. Radiation Sterilization Of Medical Devices.
From
Radiation Sterilization Of Medical Devices This document was prepared by technical committee iso/tc 198, sterilization of health care products. This part of iso 11137 describes requirements that, if met, will provide a radiation sterilization process intended to sterilize medical devices, that has. A list of all parts in the iso 11137. Radiation is a clean method of sterilization, as it does not require any chemical. Radiation Sterilization Of Medical Devices.
From
Radiation Sterilization Of Medical Devices On june 7, 2022, the fda announced it was considering a master file pilot program for premarket approval (pma) holders whose. A list of all parts in the iso 11137. This part of iso 11137 describes requirements that, if met, will provide a radiation sterilization process intended to sterilize medical devices, that has. The food and drug administration (fda) requires. Radiation Sterilization Of Medical Devices.
From
Radiation Sterilization Of Medical Devices Radiation is a clean method of sterilization, as it does not require any chemical treatment and therefore does not leave any residues behind. A list of all parts in the iso 11137. On june 7, 2022, the fda announced it was considering a master file pilot program for premarket approval (pma) holders whose. The food and drug administration (fda) requires. Radiation Sterilization Of Medical Devices.
From www.researchandmarkets.com
Radiation Sterilization of Medical Products Beyond the Basics (Recorded) Radiation Sterilization Of Medical Devices This part of iso 11137 describes requirements that, if met, will provide a radiation sterilization process intended to sterilize medical devices, that has. A list of all parts in the iso 11137. On june 7, 2022, the fda announced it was considering a master file pilot program for premarket approval (pma) holders whose. This document was prepared by technical committee. Radiation Sterilization Of Medical Devices.
From www.en-standard.eu
23/30426672 DC BS EN ISO 111371. Sterilization of health care products Radiation Sterilization Of Medical Devices Radiation is a clean method of sterilization, as it does not require any chemical treatment and therefore does not leave any residues behind. On june 7, 2022, the fda announced it was considering a master file pilot program for premarket approval (pma) holders whose. This part of iso 11137 describes requirements that, if met, will provide a radiation sterilization process. Radiation Sterilization Of Medical Devices.
From ethidelabs.com
Ethide Laboratories Liquid vs. Radiation Sterilization For Medical Radiation Sterilization Of Medical Devices This part of iso 11137 describes requirements that, if met, will provide a radiation sterilization process intended to sterilize medical devices, that has. The food and drug administration (fda) requires sterilization of all invasive medical and dental devices including syringes, surgical. On june 7, 2022, the fda announced it was considering a master file pilot program for premarket approval (pma). Radiation Sterilization Of Medical Devices.
From oxymedz.com
Sterilization Of Medical Equipment OxyMed Radiation Sterilization Of Medical Devices This part of iso 11137 describes requirements that, if met, will provide a radiation sterilization process intended to sterilize medical devices, that has. This document was prepared by technical committee iso/tc 198, sterilization of health care products. Radiation is a clean method of sterilization, as it does not require any chemical treatment and therefore does not leave any residues behind.. Radiation Sterilization Of Medical Devices.
From
Radiation Sterilization Of Medical Devices This part of iso 11137 describes requirements that, if met, will provide a radiation sterilization process intended to sterilize medical devices, that has. This document was prepared by technical committee iso/tc 198, sterilization of health care products. Radiation is a clean method of sterilization, as it does not require any chemical treatment and therefore does not leave any residues behind.. Radiation Sterilization Of Medical Devices.
From large.stanford.edu
Radiation Sterilization Radiation Sterilization Of Medical Devices A list of all parts in the iso 11137. This part of iso 11137 describes requirements that, if met, will provide a radiation sterilization process intended to sterilize medical devices, that has. Radiation is a clean method of sterilization, as it does not require any chemical treatment and therefore does not leave any residues behind. This document was prepared by. Radiation Sterilization Of Medical Devices.
From www.scribd.com
Review of Radiation Sterilization Technologies For Medical Devices Radiation Sterilization Of Medical Devices A list of all parts in the iso 11137. The food and drug administration (fda) requires sterilization of all invasive medical and dental devices including syringes, surgical. This document was prepared by technical committee iso/tc 198, sterilization of health care products. This part of iso 11137 describes requirements that, if met, will provide a radiation sterilization process intended to sterilize. Radiation Sterilization Of Medical Devices.
From
Radiation Sterilization Of Medical Devices This part of iso 11137 describes requirements that, if met, will provide a radiation sterilization process intended to sterilize medical devices, that has. On june 7, 2022, the fda announced it was considering a master file pilot program for premarket approval (pma) holders whose. Radiation is a clean method of sterilization, as it does not require any chemical treatment and. Radiation Sterilization Of Medical Devices.
From
Radiation Sterilization Of Medical Devices A list of all parts in the iso 11137. This document was prepared by technical committee iso/tc 198, sterilization of health care products. The food and drug administration (fda) requires sterilization of all invasive medical and dental devices including syringes, surgical. Radiation is a clean method of sterilization, as it does not require any chemical treatment and therefore does not. Radiation Sterilization Of Medical Devices.
From
Radiation Sterilization Of Medical Devices Radiation is a clean method of sterilization, as it does not require any chemical treatment and therefore does not leave any residues behind. A list of all parts in the iso 11137. This document was prepared by technical committee iso/tc 198, sterilization of health care products. On june 7, 2022, the fda announced it was considering a master file pilot. Radiation Sterilization Of Medical Devices.
From www.qualitymeddev.com
Gamma Sterilization Process for Medical Devices QualityMedDev Radiation Sterilization Of Medical Devices A list of all parts in the iso 11137. This part of iso 11137 describes requirements that, if met, will provide a radiation sterilization process intended to sterilize medical devices, that has. Radiation is a clean method of sterilization, as it does not require any chemical treatment and therefore does not leave any residues behind. This document was prepared by. Radiation Sterilization Of Medical Devices.
From
Radiation Sterilization Of Medical Devices On june 7, 2022, the fda announced it was considering a master file pilot program for premarket approval (pma) holders whose. A list of all parts in the iso 11137. This document was prepared by technical committee iso/tc 198, sterilization of health care products. Radiation is a clean method of sterilization, as it does not require any chemical treatment and. Radiation Sterilization Of Medical Devices.
From microbeonline.com
Radiation Sterilization Types, Mechanism, Applications • Microbe Online Radiation Sterilization Of Medical Devices The food and drug administration (fda) requires sterilization of all invasive medical and dental devices including syringes, surgical. This document was prepared by technical committee iso/tc 198, sterilization of health care products. A list of all parts in the iso 11137. On june 7, 2022, the fda announced it was considering a master file pilot program for premarket approval (pma). Radiation Sterilization Of Medical Devices.
From
Radiation Sterilization Of Medical Devices A list of all parts in the iso 11137. On june 7, 2022, the fda announced it was considering a master file pilot program for premarket approval (pma) holders whose. Radiation is a clean method of sterilization, as it does not require any chemical treatment and therefore does not leave any residues behind. This part of iso 11137 describes requirements. Radiation Sterilization Of Medical Devices.