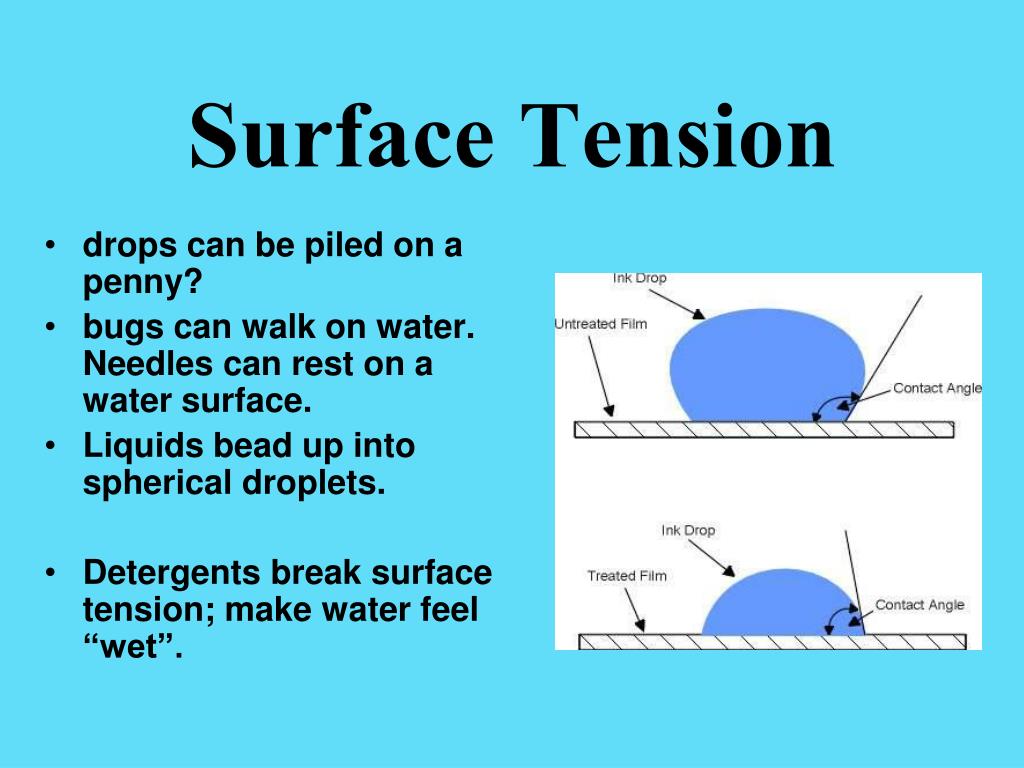
What Determines High Surface Tension . Water has a high surface tension because the water molecules. In comparison, organic liquids, such as. its cgs unit is dyne/cm. Liquids that have strong intermolecular forces, like the hydrogen. In terms of energy units, they are joules per square meter (j/m 2) in the si system and ergs/cm 2 in the cgs system. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). the surface tension of a liquid is a measure of the elastic force within the liquid's surface. 8 years ago. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension (denoted with the greek variable gamma) is defined as the ratio of the.
from www.slideserve.com
In comparison, organic liquids, such as. its cgs unit is dyne/cm. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). the surface tension of a liquid is a measure of the elastic force within the liquid's surface. 8 years ago. Liquids that have strong intermolecular forces, like the hydrogen. surface tension (denoted with the greek variable gamma) is defined as the ratio of the. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. In terms of energy units, they are joules per square meter (j/m 2) in the si system and ergs/cm 2 in the cgs system. Water has a high surface tension because the water molecules.
PPT A.P. Chemistry PowerPoint Presentation, free download ID5696562
What Determines High Surface Tension water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). 8 years ago. its cgs unit is dyne/cm. Liquids that have strong intermolecular forces, like the hydrogen. surface tension (denoted with the greek variable gamma) is defined as the ratio of the. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. In comparison, organic liquids, such as. the surface tension of a liquid is a measure of the elastic force within the liquid's surface. In terms of energy units, they are joules per square meter (j/m 2) in the si system and ergs/cm 2 in the cgs system. Water has a high surface tension because the water molecules.
From www.slideserve.com
PPT Biomedical importance of surface tension PowerPoint Presentation What Determines High Surface Tension water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. its cgs unit is dyne/cm. Water has a high surface tension because the water molecules. In comparison, organic liquids, such as.. What Determines High Surface Tension.
From www.youtube.com
Surface Tension YouTube What Determines High Surface Tension water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). 8 years ago. In comparison, organic liquids, such as. Water has a high surface tension because the water molecules. Liquids that have strong intermolecular forces, like the hydrogen. surface tension is the energy, or work, required to increase the surface area. What Determines High Surface Tension.
From www.thoughtco.com
Surface Tension Definition in Chemistry What Determines High Surface Tension Liquids that have strong intermolecular forces, like the hydrogen. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Water has a high surface tension because the water molecules. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). surface tension. What Determines High Surface Tension.
From byjus.com
On what factor does the magnitude of the surface tension of a liquid What Determines High Surface Tension Liquids that have strong intermolecular forces, like the hydrogen. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). 8 years ago. Water has a high surface tension because the water molecules. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular. What Determines High Surface Tension.
From www.expii.com
Surface Tension — Definition & Overview Expii What Determines High Surface Tension Water has a high surface tension because the water molecules. the surface tension of a liquid is a measure of the elastic force within the liquid's surface. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). In terms of energy units, they are joules per square meter (j/m 2) in the. What Determines High Surface Tension.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog What Determines High Surface Tension the surface tension of a liquid is a measure of the elastic force within the liquid's surface. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension (denoted with the greek variable gamma) is defined as the ratio of the. Water has a high surface. What Determines High Surface Tension.
From www.researchgate.net
a wetting state of surface with low and highsurfacetension fluids b What Determines High Surface Tension In comparison, organic liquids, such as. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). 8 years ago. In terms of energy units, they are joules per square meter (j/m 2) in the si system and ergs/cm 2 in the cgs system. surface tension is the energy, or work, required. What Determines High Surface Tension.
From civilchapola.com
What's Surface Tension in Fluid Mechanics Water Droplet & Soap Bubble What Determines High Surface Tension In terms of energy units, they are joules per square meter (j/m 2) in the si system and ergs/cm 2 in the cgs system. In comparison, organic liquids, such as. Water has a high surface tension because the water molecules. the surface tension of a liquid is a measure of the elastic force within the liquid's surface. Liquids that. What Determines High Surface Tension.
From www.biolinscientific.com
3 ways to measure surface tension What Determines High Surface Tension In comparison, organic liquids, such as. Liquids that have strong intermolecular forces, like the hydrogen. Water has a high surface tension because the water molecules. In terms of energy units, they are joules per square meter (j/m 2) in the si system and ergs/cm 2 in the cgs system. surface tension (denoted with the greek variable gamma) is defined. What Determines High Surface Tension.
From www.myxxgirl.com
Ppt Lesson Cohesion Adhesion Surface Tension Powerpoint My XXX Hot Girl What Determines High Surface Tension its cgs unit is dyne/cm. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension (denoted with the greek variable gamma) is defined as the ratio of the. In comparison, organic liquids, such as. 8 years ago. the surface tension of a liquid. What Determines High Surface Tension.
From www.thoughtco.com
What Is Surface Tension? Definition and Experiments What Determines High Surface Tension the surface tension of a liquid is a measure of the elastic force within the liquid's surface. Liquids that have strong intermolecular forces, like the hydrogen. Water has a high surface tension because the water molecules. 8 years ago. its cgs unit is dyne/cm. surface tension (denoted with the greek variable gamma) is defined as the. What Determines High Surface Tension.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation ID245553 What Determines High Surface Tension Liquids that have strong intermolecular forces, like the hydrogen. Water has a high surface tension because the water molecules. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). 8 years ago. the surface tension of a liquid is a measure of the elastic force within the liquid's surface. surface. What Determines High Surface Tension.
From www.sliderbase.com
Properties of Liquids Presentation Chemistry What Determines High Surface Tension surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Liquids that have strong intermolecular forces, like the hydrogen. its cgs unit is dyne/cm. the surface tension of a liquid is a measure of the elastic force within the liquid's surface. In comparison, organic liquids, such as.. What Determines High Surface Tension.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance What Determines High Surface Tension the surface tension of a liquid is a measure of the elastic force within the liquid's surface. its cgs unit is dyne/cm. Water has a high surface tension because the water molecules. 8 years ago. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). In comparison, organic liquids, such. What Determines High Surface Tension.
From www.slideserve.com
PPT Intermolecular Forces, Liquids and Solids PowerPoint Presentation What Determines High Surface Tension the surface tension of a liquid is a measure of the elastic force within the liquid's surface. 8 years ago. its cgs unit is dyne/cm. In comparison, organic liquids, such as. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Liquids that have strong intermolecular forces, like the hydrogen.. What Determines High Surface Tension.
From www.slideserve.com
PPT States of Matter PowerPoint Presentation, free download ID6036532 What Determines High Surface Tension its cgs unit is dyne/cm. In comparison, organic liquids, such as. surface tension (denoted with the greek variable gamma) is defined as the ratio of the. In terms of energy units, they are joules per square meter (j/m 2) in the si system and ergs/cm 2 in the cgs system. 8 years ago. water has a. What Determines High Surface Tension.
From www.slideserve.com
PPT Unit 4 The Hydrosphere PowerPoint Presentation, free download What Determines High Surface Tension Liquids that have strong intermolecular forces, like the hydrogen. In comparison, organic liquids, such as. Water has a high surface tension because the water molecules. In terms of energy units, they are joules per square meter (j/m 2) in the si system and ergs/cm 2 in the cgs system. its cgs unit is dyne/cm. water has a surface. What Determines High Surface Tension.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy What Determines High Surface Tension In comparison, organic liquids, such as. Liquids that have strong intermolecular forces, like the hydrogen. Water has a high surface tension because the water molecules. 8 years ago. surface tension (denoted with the greek variable gamma) is defined as the ratio of the. surface tension is the energy, or work, required to increase the surface area of. What Determines High Surface Tension.
From www.youtube.com
Surface Tension YouTube What Determines High Surface Tension In comparison, organic liquids, such as. its cgs unit is dyne/cm. surface tension (denoted with the greek variable gamma) is defined as the ratio of the. Water has a high surface tension because the water molecules. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). In terms of energy units,. What Determines High Surface Tension.
From www.slideserve.com
PPT A.P. Chemistry PowerPoint Presentation, free download ID5696562 What Determines High Surface Tension the surface tension of a liquid is a measure of the elastic force within the liquid's surface. its cgs unit is dyne/cm. surface tension (denoted with the greek variable gamma) is defined as the ratio of the. Water has a high surface tension because the water molecules. In terms of energy units, they are joules per square. What Determines High Surface Tension.
From www.inspiritvr.com
Surface Tension Study Guide Inspirit Learning Inc What Determines High Surface Tension the surface tension of a liquid is a measure of the elastic force within the liquid's surface. 8 years ago. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f).. What Determines High Surface Tension.
From www.aakash.ac.in
Surface Tension Definition, Causes, Measurement & Formula AESL What Determines High Surface Tension In terms of energy units, they are joules per square meter (j/m 2) in the si system and ergs/cm 2 in the cgs system. surface tension (denoted with the greek variable gamma) is defined as the ratio of the. In comparison, organic liquids, such as. Liquids that have strong intermolecular forces, like the hydrogen. water has a surface. What Determines High Surface Tension.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID3227401 What Determines High Surface Tension Liquids that have strong intermolecular forces, like the hydrogen. In comparison, organic liquids, such as. Water has a high surface tension because the water molecules. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension (denoted with the greek variable gamma) is defined as the ratio. What Determines High Surface Tension.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy What Determines High Surface Tension surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Water has a high surface tension because the water molecules. In comparison, organic liquids, such as. In terms of energy units, they are joules per square meter (j/m 2) in the si system and ergs/cm 2 in the cgs. What Determines High Surface Tension.
From byjus.com
Explain the surface tension phenomenon with examples. What Determines High Surface Tension In comparison, organic liquids, such as. its cgs unit is dyne/cm. Water has a high surface tension because the water molecules. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Liquids that have strong intermolecular forces, like the hydrogen. the surface tension of a liquid is. What Determines High Surface Tension.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it What Determines High Surface Tension surface tension (denoted with the greek variable gamma) is defined as the ratio of the. Water has a high surface tension because the water molecules. 8 years ago. In comparison, organic liquids, such as. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Liquids that have. What Determines High Surface Tension.
From dornshuld.chemistry.msstate.edu
1.4 Surface Tension Chemistry What Determines High Surface Tension Liquids that have strong intermolecular forces, like the hydrogen. In terms of energy units, they are joules per square meter (j/m 2) in the si system and ergs/cm 2 in the cgs system. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). surface tension (denoted with the greek variable gamma) is. What Determines High Surface Tension.
From www.slideserve.com
PPT Liquids PowerPoint Presentation, free download ID1783620 What Determines High Surface Tension In comparison, organic liquids, such as. In terms of energy units, they are joules per square meter (j/m 2) in the si system and ergs/cm 2 in the cgs system. 8 years ago. Water has a high surface tension because the water molecules. water has a surface tension of 0.07275 joule per square metre at 20 °c (68. What Determines High Surface Tension.
From www.slideserve.com
PPT Measuring the Surface Tension of Water by Light Diffraction on What Determines High Surface Tension surface tension (denoted with the greek variable gamma) is defined as the ratio of the. Water has a high surface tension because the water molecules. Liquids that have strong intermolecular forces, like the hydrogen. its cgs unit is dyne/cm. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). surface. What Determines High Surface Tension.
From funsizephysics.com
What is Surface Tension? FunsizePhysics What Determines High Surface Tension surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. In terms of energy units, they are joules per square meter (j/m 2) in the si system and ergs/cm 2 in the cgs system. water has a surface tension of 0.07275 joule per square metre at 20 °c. What Determines High Surface Tension.
From study.com
Surface Tension Definition, Calculation & Examples Video & Lesson What Determines High Surface Tension surface tension (denoted with the greek variable gamma) is defined as the ratio of the. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Liquids that have strong intermolecular forces, like the hydrogen. its cgs unit is dyne/cm. 8 years ago. water has a. What Determines High Surface Tension.
From chemwiki.ucdavis.edu
11.3 Unique Properties of Liquids Chemwiki What Determines High Surface Tension In terms of energy units, they are joules per square meter (j/m 2) in the si system and ergs/cm 2 in the cgs system. surface tension (denoted with the greek variable gamma) is defined as the ratio of the. the surface tension of a liquid is a measure of the elastic force within the liquid's surface. surface. What Determines High Surface Tension.
From www.youtube.com
Surface Tension YouTube What Determines High Surface Tension In terms of energy units, they are joules per square meter (j/m 2) in the si system and ergs/cm 2 in the cgs system. its cgs unit is dyne/cm. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension (denoted with the greek variable gamma). What Determines High Surface Tension.
From vectormine.com
Surface tension explanation vector illustration diagram VectorMine What Determines High Surface Tension In terms of energy units, they are joules per square meter (j/m 2) in the si system and ergs/cm 2 in the cgs system. In comparison, organic liquids, such as. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension (denoted with the greek variable gamma). What Determines High Surface Tension.
From www.slideserve.com
PPT Biomedical importance of surface tension PowerPoint Presentation What Determines High Surface Tension 8 years ago. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. the surface tension of a liquid is a measure of the elastic force within the liquid's surface. Liquids that have strong intermolecular forces, like the hydrogen. surface tension (denoted with the greek variable. What Determines High Surface Tension.