How Does Surface Tension Affect Force . Forces between atoms and molecules underlie the macroscopic effect called surface tension. On this basis, surface tension is often expressed as an amount of force. Various intermolecular forces, such as van der waals forces, draw the liquid particles together. Along the surface, the particles are pulled toward the. These attractive forces pull the molecules closer together and tend to minimize the surface. These attractive forces pull the molecules closer. Surface tension is also viewed as the result of forces acting in the plane of the surface and tending to minimize its area. Since these intermolecular forces vary depending on the nature of the. This effect results from the difference between the potential energy of atomic. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Forces between atoms and molecules underlie the macroscopic effect called surface tension.
from readchemistry.com
This effect results from the difference between the potential energy of atomic. Since these intermolecular forces vary depending on the nature of the. Surface tension is also viewed as the result of forces acting in the plane of the surface and tending to minimize its area. Along the surface, the particles are pulled toward the. These attractive forces pull the molecules closer. Forces between atoms and molecules underlie the macroscopic effect called surface tension. These attractive forces pull the molecules closer together and tend to minimize the surface. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Various intermolecular forces, such as van der waals forces, draw the liquid particles together. On this basis, surface tension is often expressed as an amount of force.
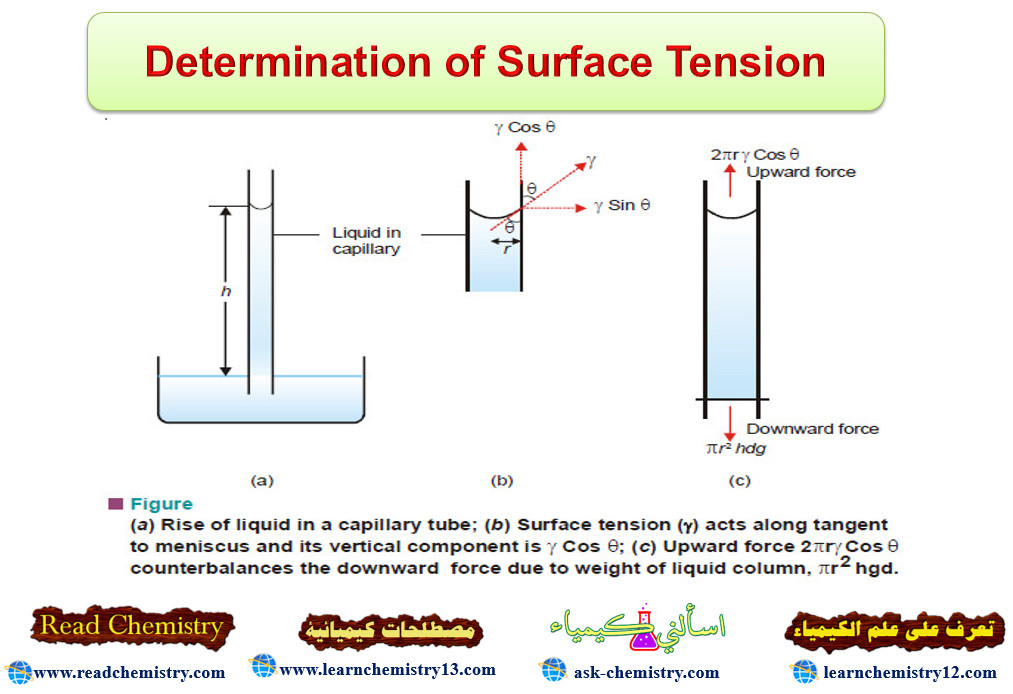
Determination of Surface Tension Read Chemistry
How Does Surface Tension Affect Force On this basis, surface tension is often expressed as an amount of force. This effect results from the difference between the potential energy of atomic. These attractive forces pull the molecules closer together and tend to minimize the surface. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. On this basis, surface tension is often expressed as an amount of force. Along the surface, the particles are pulled toward the. Since these intermolecular forces vary depending on the nature of the. These attractive forces pull the molecules closer. Various intermolecular forces, such as van der waals forces, draw the liquid particles together. Surface tension is also viewed as the result of forces acting in the plane of the surface and tending to minimize its area. Forces between atoms and molecules underlie the macroscopic effect called surface tension. Forces between atoms and molecules underlie the macroscopic effect called surface tension.
From www.dreamstime.com
Surface Tension Explanation Vector Illustration Diagram Stock Vector How Does Surface Tension Affect Force On this basis, surface tension is often expressed as an amount of force. Various intermolecular forces, such as van der waals forces, draw the liquid particles together. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. These attractive forces pull the molecules closer together and tend to minimize the. How Does Surface Tension Affect Force.
From brainly.in
How do we calculate surface tension? please explain in simple language How Does Surface Tension Affect Force On this basis, surface tension is often expressed as an amount of force. Various intermolecular forces, such as van der waals forces, draw the liquid particles together. Surface tension is also viewed as the result of forces acting in the plane of the surface and tending to minimize its area. These attractive forces pull the molecules closer together and tend. How Does Surface Tension Affect Force.
From byjus.com
Explain the surface tension phenomenon with examples. How Does Surface Tension Affect Force Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Various intermolecular forces, such as van der waals forces, draw the liquid particles together. These attractive forces pull the molecules closer. Surface tension is also viewed as the result of forces acting in the plane of the surface and tending. How Does Surface Tension Affect Force.
From www.sciencefacts.net
Surface Tension Definition, Examples, and Unit How Does Surface Tension Affect Force Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. These attractive forces pull the molecules closer together and tend to minimize the surface. This effect results from the difference between the potential energy of atomic. Forces between atoms and molecules underlie the macroscopic effect called surface tension. Surface tension. How Does Surface Tension Affect Force.
From cezpizpe.blob.core.windows.net
How Does Surface Tension Affect Heat at Felix Chavez blog How Does Surface Tension Affect Force These attractive forces pull the molecules closer. Along the surface, the particles are pulled toward the. On this basis, surface tension is often expressed as an amount of force. This effect results from the difference between the potential energy of atomic. These attractive forces pull the molecules closer together and tend to minimize the surface. Various intermolecular forces, such as. How Does Surface Tension Affect Force.
From mungfali.com
Tension Diagram How Does Surface Tension Affect Force Various intermolecular forces, such as van der waals forces, draw the liquid particles together. These attractive forces pull the molecules closer. These attractive forces pull the molecules closer together and tend to minimize the surface. Surface tension is also viewed as the result of forces acting in the plane of the surface and tending to minimize its area. This effect. How Does Surface Tension Affect Force.
From readchemistry.com
Determination of Surface Tension Read Chemistry How Does Surface Tension Affect Force Forces between atoms and molecules underlie the macroscopic effect called surface tension. This effect results from the difference between the potential energy of atomic. On this basis, surface tension is often expressed as an amount of force. Various intermolecular forces, such as van der waals forces, draw the liquid particles together. These attractive forces pull the molecules closer. Surface tension. How Does Surface Tension Affect Force.
From delprofedefisica.blogspot.com
Física Tensión superficial How Does Surface Tension Affect Force Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Forces between atoms and molecules underlie the macroscopic effect called surface tension. Various intermolecular forces, such as van der waals forces, draw the liquid particles together. Forces between atoms and molecules underlie the macroscopic effect called surface tension. These attractive. How Does Surface Tension Affect Force.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts How Does Surface Tension Affect Force Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. This effect results from the difference between the potential energy of atomic. Various intermolecular forces, such as van der waals forces, draw the liquid particles together. Surface tension is also viewed as the result of forces acting in the plane. How Does Surface Tension Affect Force.
From dxovlehoe.blob.core.windows.net
How Does Surface Tension Impact Life at Verena Cowan blog How Does Surface Tension Affect Force Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is also viewed as the result of forces acting in the plane of the surface and tending to minimize its area. Since these intermolecular forces vary depending on the nature of the. These attractive forces pull the molecules. How Does Surface Tension Affect Force.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it How Does Surface Tension Affect Force Along the surface, the particles are pulled toward the. These attractive forces pull the molecules closer together and tend to minimize the surface. Since these intermolecular forces vary depending on the nature of the. Forces between atoms and molecules underlie the macroscopic effect called surface tension. Various intermolecular forces, such as van der waals forces, draw the liquid particles together.. How Does Surface Tension Affect Force.
From upberi.com
Surface Tension Definition, Formula, Causes, Examples, and FAQs (2023) How Does Surface Tension Affect Force On this basis, surface tension is often expressed as an amount of force. Various intermolecular forces, such as van der waals forces, draw the liquid particles together. This effect results from the difference between the potential energy of atomic. These attractive forces pull the molecules closer. Forces between atoms and molecules underlie the macroscopic effect called surface tension. Forces between. How Does Surface Tension Affect Force.
From www.dreamstime.com
Surface Tension stock vector. Illustration of environment 92206295 How Does Surface Tension Affect Force On this basis, surface tension is often expressed as an amount of force. Forces between atoms and molecules underlie the macroscopic effect called surface tension. Surface tension is also viewed as the result of forces acting in the plane of the surface and tending to minimize its area. These attractive forces pull the molecules closer. Various intermolecular forces, such as. How Does Surface Tension Affect Force.
From dxovhyvyi.blob.core.windows.net
How Does Surface Tension Affect Plants at Romaine Mcewan blog How Does Surface Tension Affect Force Since these intermolecular forces vary depending on the nature of the. Surface tension is also viewed as the result of forces acting in the plane of the surface and tending to minimize its area. Various intermolecular forces, such as van der waals forces, draw the liquid particles together. On this basis, surface tension is often expressed as an amount of. How Does Surface Tension Affect Force.
From cezpizpe.blob.core.windows.net
How Does Surface Tension Affect Heat at Felix Chavez blog How Does Surface Tension Affect Force Since these intermolecular forces vary depending on the nature of the. Surface tension is also viewed as the result of forces acting in the plane of the surface and tending to minimize its area. Along the surface, the particles are pulled toward the. Forces between atoms and molecules underlie the macroscopic effect called surface tension. These attractive forces pull the. How Does Surface Tension Affect Force.
From dxovlehoe.blob.core.windows.net
How Does Surface Tension Impact Life at Verena Cowan blog How Does Surface Tension Affect Force Forces between atoms and molecules underlie the macroscopic effect called surface tension. Since these intermolecular forces vary depending on the nature of the. On this basis, surface tension is often expressed as an amount of force. This effect results from the difference between the potential energy of atomic. Surface tension is also viewed as the result of forces acting in. How Does Surface Tension Affect Force.
From www.aakash.ac.in
Surface Tension Definition, Causes, Measurement & Formula AESL How Does Surface Tension Affect Force These attractive forces pull the molecules closer together and tend to minimize the surface. Various intermolecular forces, such as van der waals forces, draw the liquid particles together. Forces between atoms and molecules underlie the macroscopic effect called surface tension. These attractive forces pull the molecules closer. Since these intermolecular forces vary depending on the nature of the. This effect. How Does Surface Tension Affect Force.
From dxovhyvyi.blob.core.windows.net
How Does Surface Tension Affect Plants at Romaine Mcewan blog How Does Surface Tension Affect Force Forces between atoms and molecules underlie the macroscopic effect called surface tension. Various intermolecular forces, such as van der waals forces, draw the liquid particles together. These attractive forces pull the molecules closer together and tend to minimize the surface. These attractive forces pull the molecules closer. Surface tension is the energy, or work, required to increase the surface area. How Does Surface Tension Affect Force.
From www.biolinscientific.com
Surface tension of water Why is it so high? How Does Surface Tension Affect Force On this basis, surface tension is often expressed as an amount of force. Various intermolecular forces, such as van der waals forces, draw the liquid particles together. This effect results from the difference between the potential energy of atomic. Forces between atoms and molecules underlie the macroscopic effect called surface tension. Surface tension is the energy, or work, required to. How Does Surface Tension Affect Force.
From bio.libretexts.org
4.5.1.3 CohesionTension Theory Biology LibreTexts How Does Surface Tension Affect Force Surface tension is also viewed as the result of forces acting in the plane of the surface and tending to minimize its area. These attractive forces pull the molecules closer together and tend to minimize the surface. This effect results from the difference between the potential energy of atomic. Since these intermolecular forces vary depending on the nature of the.. How Does Surface Tension Affect Force.
From www.aircraftsystemstech.com
Major Structural Stresses of the Aircraft How Does Surface Tension Affect Force Various intermolecular forces, such as van der waals forces, draw the liquid particles together. These attractive forces pull the molecules closer together and tend to minimize the surface. These attractive forces pull the molecules closer. This effect results from the difference between the potential energy of atomic. Surface tension is the energy, or work, required to increase the surface area. How Does Surface Tension Affect Force.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog How Does Surface Tension Affect Force This effect results from the difference between the potential energy of atomic. These attractive forces pull the molecules closer together and tend to minimize the surface. Since these intermolecular forces vary depending on the nature of the. Forces between atoms and molecules underlie the macroscopic effect called surface tension. Surface tension is the energy, or work, required to increase the. How Does Surface Tension Affect Force.
From sophisticatedthoughtscom.wordpress.com
The Science Behind Bubbles (1/3) Sophisticated Thoughts How Does Surface Tension Affect Force Forces between atoms and molecules underlie the macroscopic effect called surface tension. Forces between atoms and molecules underlie the macroscopic effect called surface tension. On this basis, surface tension is often expressed as an amount of force. Surface tension is also viewed as the result of forces acting in the plane of the surface and tending to minimize its area.. How Does Surface Tension Affect Force.
From www.slideserve.com
PPT HOW SOAP WORKS PowerPoint Presentation, free download ID1114540 How Does Surface Tension Affect Force Forces between atoms and molecules underlie the macroscopic effect called surface tension. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Various intermolecular forces, such as van der waals forces, draw the liquid particles together. These attractive forces pull the molecules closer together and tend to minimize the surface.. How Does Surface Tension Affect Force.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy How Does Surface Tension Affect Force Forces between atoms and molecules underlie the macroscopic effect called surface tension. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. These attractive forces pull the molecules closer together and tend to minimize the surface. Since these intermolecular forces vary depending on the nature of the. These attractive forces. How Does Surface Tension Affect Force.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance How Does Surface Tension Affect Force Forces between atoms and molecules underlie the macroscopic effect called surface tension. On this basis, surface tension is often expressed as an amount of force. Along the surface, the particles are pulled toward the. Various intermolecular forces, such as van der waals forces, draw the liquid particles together. Since these intermolecular forces vary depending on the nature of the. These. How Does Surface Tension Affect Force.
From www.aakash.ac.in
Surface Tension Definition, Causes, Measurement & Formula AESL How Does Surface Tension Affect Force Forces between atoms and molecules underlie the macroscopic effect called surface tension. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Along the surface, the particles are pulled toward the. Surface tension is also viewed as the result of forces acting in the plane of the surface and tending. How Does Surface Tension Affect Force.
From www.youtube.com
Surface Tension YouTube How Does Surface Tension Affect Force Forces between atoms and molecules underlie the macroscopic effect called surface tension. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Forces between atoms and molecules underlie the macroscopic effect called surface tension. Since these intermolecular forces vary depending on the nature of the. These attractive forces pull the. How Does Surface Tension Affect Force.
From www.youtube.com
How does Surface Tension work? YouTube How Does Surface Tension Affect Force Various intermolecular forces, such as van der waals forces, draw the liquid particles together. Since these intermolecular forces vary depending on the nature of the. Forces between atoms and molecules underlie the macroscopic effect called surface tension. This effect results from the difference between the potential energy of atomic. These attractive forces pull the molecules closer together and tend to. How Does Surface Tension Affect Force.
From www.merchantnavydecoded.com
What is Surface tension? Why does surface tension occur? Merchant How Does Surface Tension Affect Force These attractive forces pull the molecules closer together and tend to minimize the surface. Forces between atoms and molecules underlie the macroscopic effect called surface tension. Various intermolecular forces, such as van der waals forces, draw the liquid particles together. On this basis, surface tension is often expressed as an amount of force. Forces between atoms and molecules underlie the. How Does Surface Tension Affect Force.
From funsizephysics.com
What is Surface Tension? FunsizePhysics How Does Surface Tension Affect Force Forces between atoms and molecules underlie the macroscopic effect called surface tension. Various intermolecular forces, such as van der waals forces, draw the liquid particles together. Forces between atoms and molecules underlie the macroscopic effect called surface tension. Surface tension is also viewed as the result of forces acting in the plane of the surface and tending to minimize its. How Does Surface Tension Affect Force.
From loetunjhi.blob.core.windows.net
Why Does Surface Tension Happen at Stan Stewart blog How Does Surface Tension Affect Force Various intermolecular forces, such as van der waals forces, draw the liquid particles together. On this basis, surface tension is often expressed as an amount of force. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Forces between atoms and molecules underlie the macroscopic effect called surface tension. These. How Does Surface Tension Affect Force.
From www.slideserve.com
PPT States of Matter PowerPoint Presentation, free download ID6036532 How Does Surface Tension Affect Force These attractive forces pull the molecules closer. These attractive forces pull the molecules closer together and tend to minimize the surface. Along the surface, the particles are pulled toward the. Forces between atoms and molecules underlie the macroscopic effect called surface tension. Surface tension is also viewed as the result of forces acting in the plane of the surface and. How Does Surface Tension Affect Force.
From askfilo.com
Surface tension does not vary with Filo How Does Surface Tension Affect Force Surface tension is also viewed as the result of forces acting in the plane of the surface and tending to minimize its area. On this basis, surface tension is often expressed as an amount of force. Forces between atoms and molecules underlie the macroscopic effect called surface tension. Forces between atoms and molecules underlie the macroscopic effect called surface tension.. How Does Surface Tension Affect Force.
From www.chegg.com
Solved a) [20 points] Find the angle of contact in Figure 1 How Does Surface Tension Affect Force These attractive forces pull the molecules closer together and tend to minimize the surface. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. On this basis, surface tension is often expressed as an amount of force. Forces between atoms and molecules underlie the macroscopic effect called surface tension. Surface. How Does Surface Tension Affect Force.