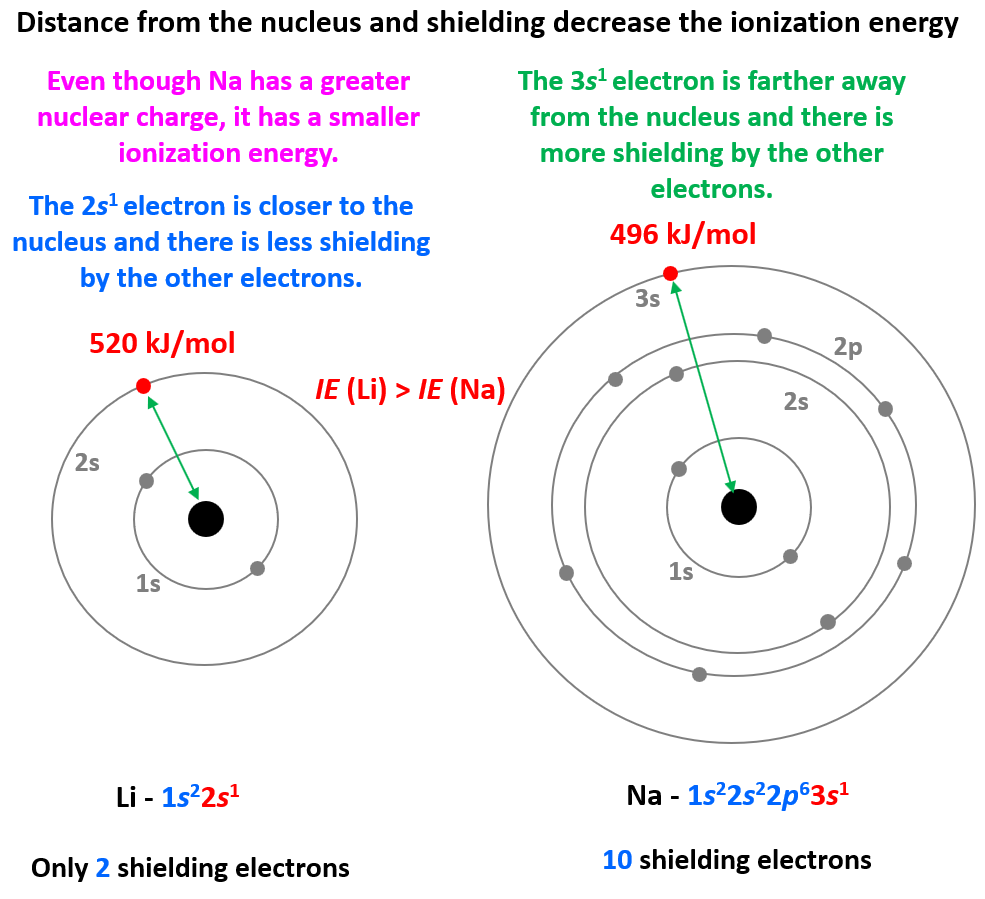
What Is Electron Shielding A Level Chemistry . Filled energy levels can shield (mask) the effect of the nuclear charge causing the outer electrons to be less attracted to the nucleus;. This means there is a greater electrostatic attraction which pulls the outer electrons more tightly in towards the nucleus,. Hence, the nucleus has less grip on the outer electrons and are shielded from them. The concept called electron shielding involves the outer electrons are partially shielded from the attractive force of the protons in the nucleus by. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. More electron shells means more electron shielding so there i s weaker attraction between outer shell electrons and the.
from general.chemistrysteps.com
Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. The concept called electron shielding involves the outer electrons are partially shielded from the attractive force of the protons in the nucleus by. Hence, the nucleus has less grip on the outer electrons and are shielded from them. Filled energy levels can shield (mask) the effect of the nuclear charge causing the outer electrons to be less attracted to the nucleus;. More electron shells means more electron shielding so there i s weaker attraction between outer shell electrons and the. This means there is a greater electrostatic attraction which pulls the outer electrons more tightly in towards the nucleus,.
Ionization energy Chemistry Steps
What Is Electron Shielding A Level Chemistry This means there is a greater electrostatic attraction which pulls the outer electrons more tightly in towards the nucleus,. Hence, the nucleus has less grip on the outer electrons and are shielded from them. More electron shells means more electron shielding so there i s weaker attraction between outer shell electrons and the. This means there is a greater electrostatic attraction which pulls the outer electrons more tightly in towards the nucleus,. The concept called electron shielding involves the outer electrons are partially shielded from the attractive force of the protons in the nucleus by. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Filled energy levels can shield (mask) the effect of the nuclear charge causing the outer electrons to be less attracted to the nucleus;.
From www.nuclear-power.com
Shielding of Alpha Radiation Types & Uses What Is Electron Shielding A Level Chemistry The concept called electron shielding involves the outer electrons are partially shielded from the attractive force of the protons in the nucleus by. Filled energy levels can shield (mask) the effect of the nuclear charge causing the outer electrons to be less attracted to the nucleus;. Hence, the nucleus has less grip on the outer electrons and are shielded from. What Is Electron Shielding A Level Chemistry.
From byjus.com
What is shielding and deshielding in NMR? Give an example? What Is Electron Shielding A Level Chemistry Filled energy levels can shield (mask) the effect of the nuclear charge causing the outer electrons to be less attracted to the nucleus;. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. More electron shells means more electron shielding so there i s weaker attraction between outer shell electrons and the. This means. What Is Electron Shielding A Level Chemistry.
From www.expii.com
Valence Electrons — Definition & Importance Expii What Is Electron Shielding A Level Chemistry The concept called electron shielding involves the outer electrons are partially shielded from the attractive force of the protons in the nucleus by. More electron shells means more electron shielding so there i s weaker attraction between outer shell electrons and the. Filled energy levels can shield (mask) the effect of the nuclear charge causing the outer electrons to be. What Is Electron Shielding A Level Chemistry.
From www.slideserve.com
PPT Lesson objectives • Define first ionisation energy and successive What Is Electron Shielding A Level Chemistry More electron shells means more electron shielding so there i s weaker attraction between outer shell electrons and the. Hence, the nucleus has less grip on the outer electrons and are shielded from them. Filled energy levels can shield (mask) the effect of the nuclear charge causing the outer electrons to be less attracted to the nucleus;. The concept called. What Is Electron Shielding A Level Chemistry.
From scienceinfo.com
Shielding effect What Is Electron Shielding A Level Chemistry Hence, the nucleus has less grip on the outer electrons and are shielded from them. More electron shells means more electron shielding so there i s weaker attraction between outer shell electrons and the. Filled energy levels can shield (mask) the effect of the nuclear charge causing the outer electrons to be less attracted to the nucleus;. This means there. What Is Electron Shielding A Level Chemistry.
From www.slideserve.com
PPT Chapter 14 Chemical Periodicity PowerPoint Presentation, free What Is Electron Shielding A Level Chemistry Filled energy levels can shield (mask) the effect of the nuclear charge causing the outer electrons to be less attracted to the nucleus;. Hence, the nucleus has less grip on the outer electrons and are shielded from them. More electron shells means more electron shielding so there i s weaker attraction between outer shell electrons and the. Shielding refers to. What Is Electron Shielding A Level Chemistry.
From www.carlsonstockart.com
Electron Energy Levels of Atoms Carlson Stock Art What Is Electron Shielding A Level Chemistry Filled energy levels can shield (mask) the effect of the nuclear charge causing the outer electrons to be less attracted to the nucleus;. More electron shells means more electron shielding so there i s weaker attraction between outer shell electrons and the. This means there is a greater electrostatic attraction which pulls the outer electrons more tightly in towards the. What Is Electron Shielding A Level Chemistry.
From ceuwfxbf.blob.core.windows.net
What Does A Shielding Electron at Pamela Mccarthy blog What Is Electron Shielding A Level Chemistry More electron shells means more electron shielding so there i s weaker attraction between outer shell electrons and the. This means there is a greater electrostatic attraction which pulls the outer electrons more tightly in towards the nucleus,. Hence, the nucleus has less grip on the outer electrons and are shielded from them. Shielding refers to the core electrons repelling. What Is Electron Shielding A Level Chemistry.
From wou.edu
CH104 Chapter 2 Atoms and The Periodic Table Chemistry What Is Electron Shielding A Level Chemistry Hence, the nucleus has less grip on the outer electrons and are shielded from them. This means there is a greater electrostatic attraction which pulls the outer electrons more tightly in towards the nucleus,. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. The concept called electron shielding involves the outer electrons are. What Is Electron Shielding A Level Chemistry.
From simpleenglishchemistry.blogspot.com
Simple English Chemistry Atomic Size/Atomic Radius, Electronegativity What Is Electron Shielding A Level Chemistry This means there is a greater electrostatic attraction which pulls the outer electrons more tightly in towards the nucleus,. More electron shells means more electron shielding so there i s weaker attraction between outer shell electrons and the. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Filled energy levels can shield (mask). What Is Electron Shielding A Level Chemistry.
From www.studocu.com
Shielding of Electrons Chemistry V Lab Studocu What Is Electron Shielding A Level Chemistry Filled energy levels can shield (mask) the effect of the nuclear charge causing the outer electrons to be less attracted to the nucleus;. This means there is a greater electrostatic attraction which pulls the outer electrons more tightly in towards the nucleus,. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. The concept. What Is Electron Shielding A Level Chemistry.
From socratic.org
How are shielding effect and atomic radius related? Socratic What Is Electron Shielding A Level Chemistry Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. More electron shells means more electron shielding so there i s weaker attraction between outer shell electrons and the. Filled energy levels can shield (mask) the effect of the nuclear charge causing the outer electrons to be less attracted to the nucleus;. This means. What Is Electron Shielding A Level Chemistry.
From www.slideserve.com
PPT Periodic Trends PowerPoint Presentation, free download ID3652853 What Is Electron Shielding A Level Chemistry Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Filled energy levels can shield (mask) the effect of the nuclear charge causing the outer electrons to be less attracted to the nucleus;. More electron shells means more electron shielding so there i s weaker attraction between outer shell electrons and the. This means. What Is Electron Shielding A Level Chemistry.
From www.youtube.com
Shielding Effect and Effective Nuclear Charge YouTube What Is Electron Shielding A Level Chemistry Filled energy levels can shield (mask) the effect of the nuclear charge causing the outer electrons to be less attracted to the nucleus;. Hence, the nucleus has less grip on the outer electrons and are shielded from them. The concept called electron shielding involves the outer electrons are partially shielded from the attractive force of the protons in the nucleus. What Is Electron Shielding A Level Chemistry.
From alevelchemistry.co.uk
Electron Structure ALevel Chemistry Revision Notes What Is Electron Shielding A Level Chemistry This means there is a greater electrostatic attraction which pulls the outer electrons more tightly in towards the nucleus,. Filled energy levels can shield (mask) the effect of the nuclear charge causing the outer electrons to be less attracted to the nucleus;. Hence, the nucleus has less grip on the outer electrons and are shielded from them. The concept called. What Is Electron Shielding A Level Chemistry.
From wisc.pb.unizin.org
M7Q8 Core and Valence Electrons, Shielding, Zeff Chem 103/104 What Is Electron Shielding A Level Chemistry Hence, the nucleus has less grip on the outer electrons and are shielded from them. Filled energy levels can shield (mask) the effect of the nuclear charge causing the outer electrons to be less attracted to the nucleus;. The concept called electron shielding involves the outer electrons are partially shielded from the attractive force of the protons in the nucleus. What Is Electron Shielding A Level Chemistry.
From ceuwfxbf.blob.core.windows.net
What Does A Shielding Electron at Pamela Mccarthy blog What Is Electron Shielding A Level Chemistry Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Filled energy levels can shield (mask) the effect of the nuclear charge causing the outer electrons to be less attracted to the nucleus;. The concept called electron shielding involves the outer electrons are partially shielded from the attractive force of the protons in the. What Is Electron Shielding A Level Chemistry.
From slideplayer.com
Periodic Trends Chemistry I. ppt download What Is Electron Shielding A Level Chemistry This means there is a greater electrostatic attraction which pulls the outer electrons more tightly in towards the nucleus,. Filled energy levels can shield (mask) the effect of the nuclear charge causing the outer electrons to be less attracted to the nucleus;. More electron shells means more electron shielding so there i s weaker attraction between outer shell electrons and. What Is Electron Shielding A Level Chemistry.
From www.sciencesfp.com
Electronic structure of matter. San Francisco de Paula, Science What Is Electron Shielding A Level Chemistry Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. This means there is a greater electrostatic attraction which pulls the outer electrons more tightly in towards the nucleus,. More electron shells means more electron shielding so there i s weaker attraction between outer shell electrons and the. Filled energy levels can shield (mask). What Is Electron Shielding A Level Chemistry.
From chemistnotes.com
Shielding Effect or Screening Effect Definition, Factors Affecting What Is Electron Shielding A Level Chemistry Filled energy levels can shield (mask) the effect of the nuclear charge causing the outer electrons to be less attracted to the nucleus;. The concept called electron shielding involves the outer electrons are partially shielded from the attractive force of the protons in the nucleus by. Hence, the nucleus has less grip on the outer electrons and are shielded from. What Is Electron Shielding A Level Chemistry.
From www.thesciencehive.co.uk
Electron Structure* — the science sauce What Is Electron Shielding A Level Chemistry Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. The concept called electron shielding involves the outer electrons are partially shielded from the attractive force of the protons in the nucleus by. This means there is a greater electrostatic attraction which pulls the outer electrons more tightly in towards the nucleus,. Filled energy. What Is Electron Shielding A Level Chemistry.
From slidesharetrick.blogspot.com
What Is Electron Shielding slidesharetrick What Is Electron Shielding A Level Chemistry Hence, the nucleus has less grip on the outer electrons and are shielded from them. This means there is a greater electrostatic attraction which pulls the outer electrons more tightly in towards the nucleus,. Filled energy levels can shield (mask) the effect of the nuclear charge causing the outer electrons to be less attracted to the nucleus;. More electron shells. What Is Electron Shielding A Level Chemistry.
From ar.inspiredpencil.com
Electron Shielding What Is Electron Shielding A Level Chemistry Hence, the nucleus has less grip on the outer electrons and are shielded from them. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. This means there is a greater electrostatic attraction which pulls the outer electrons more tightly in towards the nucleus,. The concept called electron shielding involves the outer electrons are. What Is Electron Shielding A Level Chemistry.
From www.slideserve.com
PPT The Periodic Table PowerPoint Presentation, free download ID What Is Electron Shielding A Level Chemistry More electron shells means more electron shielding so there i s weaker attraction between outer shell electrons and the. The concept called electron shielding involves the outer electrons are partially shielded from the attractive force of the protons in the nucleus by. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Filled energy. What Is Electron Shielding A Level Chemistry.
From www.youtube.com
S3.1.3 Electron shielding and effective nuclear charge YouTube What Is Electron Shielding A Level Chemistry Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. This means there is a greater electrostatic attraction which pulls the outer electrons more tightly in towards the nucleus,. More electron shells means more electron shielding so there i s weaker attraction between outer shell electrons and the. Filled energy levels can shield (mask). What Is Electron Shielding A Level Chemistry.
From www.youtube.com
Shielding Effect in the Periodic Table Chemistry YouTube What Is Electron Shielding A Level Chemistry This means there is a greater electrostatic attraction which pulls the outer electrons more tightly in towards the nucleus,. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. More electron shells means more electron shielding so there i s weaker attraction between outer shell electrons and the. Hence, the nucleus has less grip. What Is Electron Shielding A Level Chemistry.
From www.linstitute.net
CIE A Level Chemistry复习笔记1.3.1 Electronegativity翰林国际教育 What Is Electron Shielding A Level Chemistry Hence, the nucleus has less grip on the outer electrons and are shielded from them. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Filled energy levels can shield (mask) the effect of the nuclear charge causing the outer electrons to be less attracted to the nucleus;. The concept called electron shielding involves. What Is Electron Shielding A Level Chemistry.
From surfguppy.com
What is Electronegativity? What Is Electron Shielding A Level Chemistry The concept called electron shielding involves the outer electrons are partially shielded from the attractive force of the protons in the nucleus by. Hence, the nucleus has less grip on the outer electrons and are shielded from them. Filled energy levels can shield (mask) the effect of the nuclear charge causing the outer electrons to be less attracted to the. What Is Electron Shielding A Level Chemistry.
From www.showme.com
Electron shielding Science, Chemistry, Electrons, electron shielding What Is Electron Shielding A Level Chemistry The concept called electron shielding involves the outer electrons are partially shielded from the attractive force of the protons in the nucleus by. Filled energy levels can shield (mask) the effect of the nuclear charge causing the outer electrons to be less attracted to the nucleus;. This means there is a greater electrostatic attraction which pulls the outer electrons more. What Is Electron Shielding A Level Chemistry.
From dxoegqbbm.blob.core.windows.net
Electron Shielding Effect Ionization Energy at Elizabeth Shank blog What Is Electron Shielding A Level Chemistry Filled energy levels can shield (mask) the effect of the nuclear charge causing the outer electrons to be less attracted to the nucleus;. More electron shells means more electron shielding so there i s weaker attraction between outer shell electrons and the. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. The concept. What Is Electron Shielding A Level Chemistry.
From general.chemistrysteps.com
Ionization energy Chemistry Steps What Is Electron Shielding A Level Chemistry More electron shells means more electron shielding so there i s weaker attraction between outer shell electrons and the. The concept called electron shielding involves the outer electrons are partially shielded from the attractive force of the protons in the nucleus by. Hence, the nucleus has less grip on the outer electrons and are shielded from them. This means there. What Is Electron Shielding A Level Chemistry.
From www.youtube.com
Electron shielding YouTube What Is Electron Shielding A Level Chemistry Filled energy levels can shield (mask) the effect of the nuclear charge causing the outer electrons to be less attracted to the nucleus;. This means there is a greater electrostatic attraction which pulls the outer electrons more tightly in towards the nucleus,. More electron shells means more electron shielding so there i s weaker attraction between outer shell electrons and. What Is Electron Shielding A Level Chemistry.
From en.m.wikipedia.org
FilePeriodic table of elements showing electron shells.png Wikipedia What Is Electron Shielding A Level Chemistry The concept called electron shielding involves the outer electrons are partially shielded from the attractive force of the protons in the nucleus by. This means there is a greater electrostatic attraction which pulls the outer electrons more tightly in towards the nucleus,. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. More electron. What Is Electron Shielding A Level Chemistry.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID1951146 What Is Electron Shielding A Level Chemistry The concept called electron shielding involves the outer electrons are partially shielded from the attractive force of the protons in the nucleus by. This means there is a greater electrostatic attraction which pulls the outer electrons more tightly in towards the nucleus,. Filled energy levels can shield (mask) the effect of the nuclear charge causing the outer electrons to be. What Is Electron Shielding A Level Chemistry.
From www.slideserve.com
PPT The Periodic Table and Physical Properties PowerPoint What Is Electron Shielding A Level Chemistry Filled energy levels can shield (mask) the effect of the nuclear charge causing the outer electrons to be less attracted to the nucleus;. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Hence, the nucleus has less grip on the outer electrons and are shielded from them. This means there is a greater. What Is Electron Shielding A Level Chemistry.