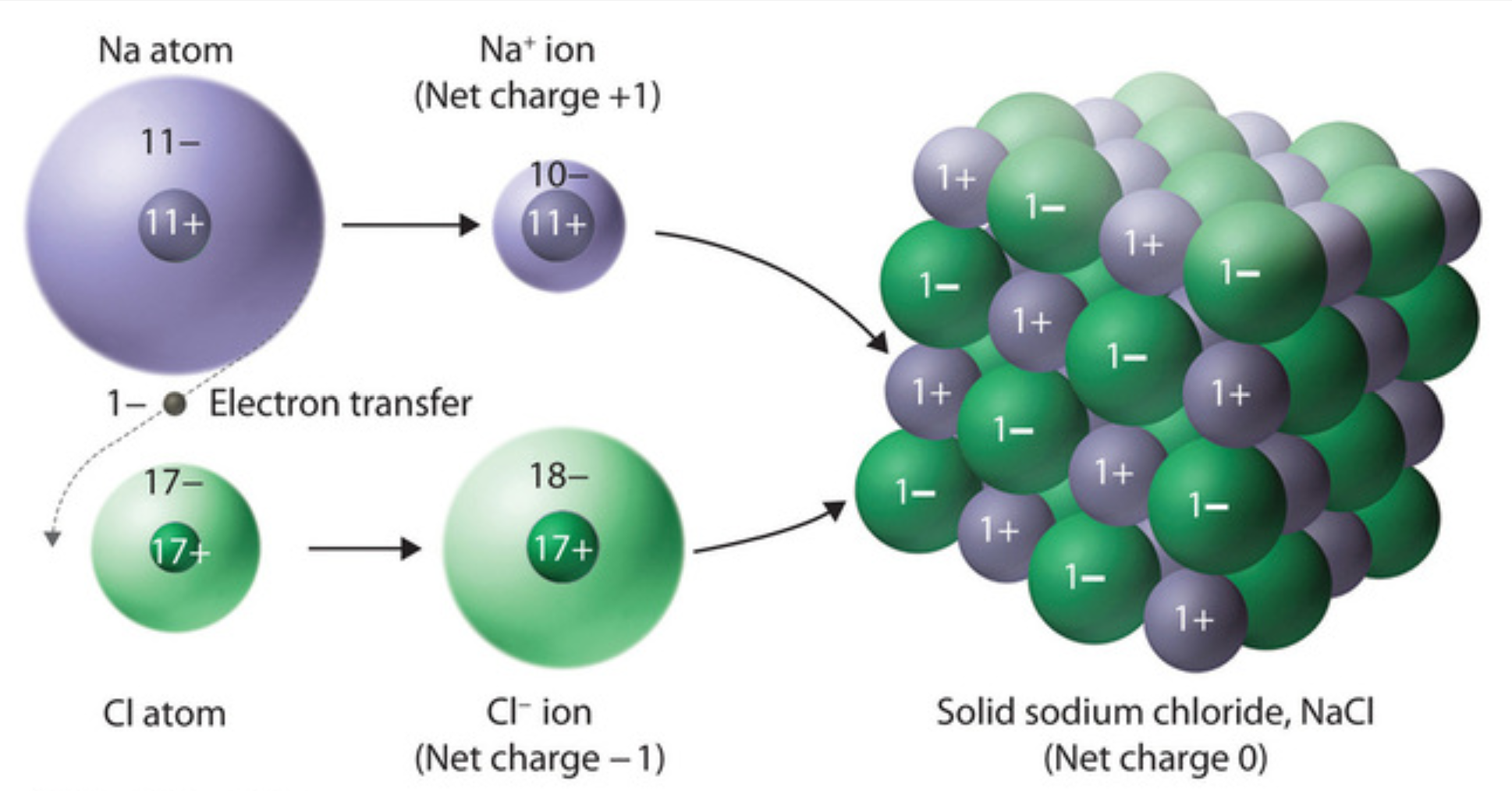
Chlorine Ion Formed . For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +, and each chlorine atom in a. The formation of a chloride ion. On the right, the chloride ion has 18 electrons and has a 1− charge. A chlorine atom (cl) gains one. A neutral chlorine atom has seven electrons in its outermost shell. This fills the chlorine atom's outer shell, making it electronically stable. To this end, chlorides are widely defined as any material containing chlorine. A chloride ion forms when a chlorine atom gains an electron. On the left, the chlorine atom has 17 electrons. A chloride ion is a negatively charged ion i.e. Only one more electron is needed to achieve an octet in chlorine’s valence shell. Learn how and why ions are formed by learning what happens when sodium and chlorine are combined to create salt. Anion, which has one unit of negative charge.
from chem.libretexts.org
On the right, the chloride ion has 18 electrons and has a 1− charge. A chlorine atom (cl) gains one. The formation of a chloride ion. Only one more electron is needed to achieve an octet in chlorine’s valence shell. On the left, the chlorine atom has 17 electrons. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +, and each chlorine atom in a. Learn how and why ions are formed by learning what happens when sodium and chlorine are combined to create salt. To this end, chlorides are widely defined as any material containing chlorine. Anion, which has one unit of negative charge. A chloride ion forms when a chlorine atom gains an electron.
4.3 The Reaction of Sodium with Chlorine Chemistry LibreTexts
Chlorine Ion Formed A chloride ion forms when a chlorine atom gains an electron. On the left, the chlorine atom has 17 electrons. A neutral chlorine atom has seven electrons in its outermost shell. On the right, the chloride ion has 18 electrons and has a 1− charge. This fills the chlorine atom's outer shell, making it electronically stable. A chlorine atom (cl) gains one. Only one more electron is needed to achieve an octet in chlorine’s valence shell. Learn how and why ions are formed by learning what happens when sodium and chlorine are combined to create salt. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +, and each chlorine atom in a. A chloride ion forms when a chlorine atom gains an electron. To this end, chlorides are widely defined as any material containing chlorine. A chloride ion is a negatively charged ion i.e. Anion, which has one unit of negative charge. The formation of a chloride ion.
From brainly.in
draw atomic structure of chlorine Brainly.in Chlorine Ion Formed On the right, the chloride ion has 18 electrons and has a 1− charge. A chloride ion forms when a chlorine atom gains an electron. Anion, which has one unit of negative charge. A chlorine atom (cl) gains one. A neutral chlorine atom has seven electrons in its outermost shell. To this end, chlorides are widely defined as any material. Chlorine Ion Formed.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica Chlorine Ion Formed On the right, the chloride ion has 18 electrons and has a 1− charge. To this end, chlorides are widely defined as any material containing chlorine. A neutral chlorine atom has seven electrons in its outermost shell. A chlorine atom (cl) gains one. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one. Chlorine Ion Formed.
From oerpub.github.io
The top panel of this figure shows the orbit model of a sodium atom and Chlorine Ion Formed On the right, the chloride ion has 18 electrons and has a 1− charge. A neutral chlorine atom has seven electrons in its outermost shell. A chloride ion is a negatively charged ion i.e. On the left, the chlorine atom has 17 electrons. Anion, which has one unit of negative charge. To this end, chlorides are widely defined as any. Chlorine Ion Formed.
From www.youtube.com
AQA Further Reactions of Chlorine YouTube Chlorine Ion Formed A chlorine atom (cl) gains one. Anion, which has one unit of negative charge. A neutral chlorine atom has seven electrons in its outermost shell. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +, and each chlorine atom in a. This fills the chlorine. Chlorine Ion Formed.
From brainly.in
Draw the ionic bond formation of Calcium Chloride... Brainly.in Chlorine Ion Formed To this end, chlorides are widely defined as any material containing chlorine. This fills the chlorine atom's outer shell, making it electronically stable. A neutral chlorine atom has seven electrons in its outermost shell. On the right, the chloride ion has 18 electrons and has a 1− charge. Learn how and why ions are formed by learning what happens when. Chlorine Ion Formed.
From brainly.com
show a diagram of how ions are formed when a sodium atom comes into Chlorine Ion Formed A chlorine atom (cl) gains one. This fills the chlorine atom's outer shell, making it electronically stable. The formation of a chloride ion. On the left, the chlorine atom has 17 electrons. A chloride ion forms when a chlorine atom gains an electron. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one. Chlorine Ion Formed.
From www.masterorganicchemistry.com
Formation of chlorohydrins from alkenes using water and Cl2 Master Chlorine Ion Formed Only one more electron is needed to achieve an octet in chlorine’s valence shell. A chloride ion forms when a chlorine atom gains an electron. On the left, the chlorine atom has 17 electrons. Learn how and why ions are formed by learning what happens when sodium and chlorine are combined to create salt. A neutral chlorine atom has seven. Chlorine Ion Formed.
From www.goodscience.com.au
Formation of Ions and Ionic Compounds Good Science Chlorine Ion Formed A neutral chlorine atom has seven electrons in its outermost shell. The formation of a chloride ion. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +, and each chlorine atom in a. A chloride ion is a negatively charged ion i.e. A chlorine atom. Chlorine Ion Formed.
From exoygyiwi.blob.core.windows.net
Chlorine Positive Or Negative Charge at John Lea blog Chlorine Ion Formed On the right, the chloride ion has 18 electrons and has a 1− charge. This fills the chlorine atom's outer shell, making it electronically stable. A neutral chlorine atom has seven electrons in its outermost shell. Only one more electron is needed to achieve an octet in chlorine’s valence shell. For example, when each sodium atom in a sample of. Chlorine Ion Formed.
From www.gauthmath.com
Solved Magnesium and chlorine react to form an ionic compound. What is Chlorine Ion Formed Learn how and why ions are formed by learning what happens when sodium and chlorine are combined to create salt. The formation of a chloride ion. A chloride ion forms when a chlorine atom gains an electron. On the right, the chloride ion has 18 electrons and has a 1− charge. For example, when each sodium atom in a sample. Chlorine Ion Formed.
From newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Chlorine Ion Formed Anion, which has one unit of negative charge. A chloride ion is a negatively charged ion i.e. To this end, chlorides are widely defined as any material containing chlorine. Learn how and why ions are formed by learning what happens when sodium and chlorine are combined to create salt. Only one more electron is needed to achieve an octet in. Chlorine Ion Formed.
From brainly.in
This question is about metal compounds. (a) Lithium reacts with Chlorine Ion Formed To this end, chlorides are widely defined as any material containing chlorine. Only one more electron is needed to achieve an octet in chlorine’s valence shell. A chloride ion forms when a chlorine atom gains an electron. A chloride ion is a negatively charged ion i.e. This fills the chlorine atom's outer shell, making it electronically stable. The formation of. Chlorine Ion Formed.
From postimg.cc
6 ionic bonding 01 — Postimages Chlorine Ion Formed For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +, and each chlorine atom in a. Only one more electron is needed to achieve an octet in chlorine’s valence shell. A chloride ion forms when a chlorine atom gains an electron. A chloride ion is. Chlorine Ion Formed.
From www.slideserve.com
PPT Chapter 6 Ionic Compounds PowerPoint Presentation, free Chlorine Ion Formed On the right, the chloride ion has 18 electrons and has a 1− charge. On the left, the chlorine atom has 17 electrons. The formation of a chloride ion. A chloride ion is a negatively charged ion i.e. This fills the chlorine atom's outer shell, making it electronically stable. Learn how and why ions are formed by learning what happens. Chlorine Ion Formed.
From joijfpoqz.blob.core.windows.net
Formation Of Table Salt Formula at John Canada blog Chlorine Ion Formed A neutral chlorine atom has seven electrons in its outermost shell. To this end, chlorides are widely defined as any material containing chlorine. A chlorine atom (cl) gains one. Learn how and why ions are formed by learning what happens when sodium and chlorine are combined to create salt. A chloride ion forms when a chlorine atom gains an electron.. Chlorine Ion Formed.
From dxopaibhs.blob.core.windows.net
A Chlorine Atom Gains An Electron. What Is The Resulting Particle at Chlorine Ion Formed The formation of a chloride ion. To this end, chlorides are widely defined as any material containing chlorine. A chloride ion forms when a chlorine atom gains an electron. A neutral chlorine atom has seven electrons in its outermost shell. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form. Chlorine Ion Formed.
From quizizz.com
Properties of Ionic Compounds Chemistry Quiz Quizizz Chlorine Ion Formed To this end, chlorides are widely defined as any material containing chlorine. A chloride ion is a negatively charged ion i.e. A chloride ion forms when a chlorine atom gains an electron. On the left, the chlorine atom has 17 electrons. Only one more electron is needed to achieve an octet in chlorine’s valence shell. Anion, which has one unit. Chlorine Ion Formed.
From ecurrencythailand.com
When An Atom Of Chlorine Forms An Ion The Atom? The 8 New Answer Chlorine Ion Formed A chlorine atom (cl) gains one. Anion, which has one unit of negative charge. On the right, the chloride ion has 18 electrons and has a 1− charge. To this end, chlorides are widely defined as any material containing chlorine. The formation of a chloride ion. Only one more electron is needed to achieve an octet in chlorine’s valence shell.. Chlorine Ion Formed.
From www2.victoriacollege.edu
formation of ionic bonds Chlorine Ion Formed A chloride ion forms when a chlorine atom gains an electron. Anion, which has one unit of negative charge. On the left, the chlorine atom has 17 electrons. The formation of a chloride ion. This fills the chlorine atom's outer shell, making it electronically stable. A chloride ion is a negatively charged ion i.e. To this end, chlorides are widely. Chlorine Ion Formed.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Chlorine Ion Formed On the left, the chlorine atom has 17 electrons. Anion, which has one unit of negative charge. A neutral chlorine atom has seven electrons in its outermost shell. On the right, the chloride ion has 18 electrons and has a 1− charge. A chlorine atom (cl) gains one. Only one more electron is needed to achieve an octet in chlorine’s. Chlorine Ion Formed.
From infraredforhealth.com
How Ions Are Formed? Infrared for Health Chlorine Ion Formed A neutral chlorine atom has seven electrons in its outermost shell. The formation of a chloride ion. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +, and each chlorine atom in a. Only one more electron is needed to achieve an octet in chlorine’s. Chlorine Ion Formed.
From www.animalia-life.club
Electron Configuration For Chlorine Chlorine Ion Formed This fills the chlorine atom's outer shell, making it electronically stable. Only one more electron is needed to achieve an octet in chlorine’s valence shell. The formation of a chloride ion. Anion, which has one unit of negative charge. On the right, the chloride ion has 18 electrons and has a 1− charge. For example, when each sodium atom in. Chlorine Ion Formed.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures Chlorine Ion Formed On the left, the chlorine atom has 17 electrons. A chloride ion is a negatively charged ion i.e. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +, and each chlorine atom in a. Learn how and why ions are formed by learning what happens. Chlorine Ion Formed.
From www.sciencenewsforstudents.org
Explainer Ions and radicals in our world Science News for Students Chlorine Ion Formed Anion, which has one unit of negative charge. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +, and each chlorine atom in a. A chlorine atom (cl) gains one. On the left, the chlorine atom has 17 electrons. Only one more electron is needed. Chlorine Ion Formed.
From byjus.com
Sodium Chloride Preparation, Properties, Structure & Uses Byju's Chlorine Ion Formed A chloride ion is a negatively charged ion i.e. The formation of a chloride ion. A chloride ion forms when a chlorine atom gains an electron. On the left, the chlorine atom has 17 electrons. To this end, chlorides are widely defined as any material containing chlorine. A chlorine atom (cl) gains one. Anion, which has one unit of negative. Chlorine Ion Formed.
From blog.chloramineconsulting.com
Pool Water Chemistry, Part 2 Combined Chlorine Chlorine Ion Formed A chloride ion is a negatively charged ion i.e. A chloride ion forms when a chlorine atom gains an electron. To this end, chlorides are widely defined as any material containing chlorine. Only one more electron is needed to achieve an octet in chlorine’s valence shell. For example, when each sodium atom in a sample of sodium metal (group 1). Chlorine Ion Formed.
From www.alamy.com
Sodium Chloride ionic bond formation. NaCl structure. Sodium and Chlorine Ion Formed On the right, the chloride ion has 18 electrons and has a 1− charge. A neutral chlorine atom has seven electrons in its outermost shell. Learn how and why ions are formed by learning what happens when sodium and chlorine are combined to create salt. A chloride ion is a negatively charged ion i.e. A chlorine atom (cl) gains one.. Chlorine Ion Formed.
From www.alamy.com
Diagram to show ionic bonding in sodium chloride Stock Vector Image Chlorine Ion Formed This fills the chlorine atom's outer shell, making it electronically stable. A chloride ion is a negatively charged ion i.e. Anion, which has one unit of negative charge. On the right, the chloride ion has 18 electrons and has a 1− charge. Learn how and why ions are formed by learning what happens when sodium and chlorine are combined to. Chlorine Ion Formed.
From www.dreamstime.com
Ionic Bonding in a Solid Sodium Chloride Crystal Stock Vector Chlorine Ion Formed Anion, which has one unit of negative charge. A chlorine atom (cl) gains one. For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +, and each chlorine atom in a. Learn how and why ions are formed by learning what happens when sodium and chlorine. Chlorine Ion Formed.
From basichemistry.blogspot.com
Basic Chemistry Ions, Cations, and Anions Chlorine Ion Formed On the right, the chloride ion has 18 electrons and has a 1− charge. The formation of a chloride ion. Learn how and why ions are formed by learning what happens when sodium and chlorine are combined to create salt. A chloride ion forms when a chlorine atom gains an electron. Only one more electron is needed to achieve an. Chlorine Ion Formed.
From www.chegg.com
Solved For the following elements, identify the charge of Chlorine Ion Formed Learn how and why ions are formed by learning what happens when sodium and chlorine are combined to create salt. A chlorine atom (cl) gains one. A neutral chlorine atom has seven electrons in its outermost shell. Anion, which has one unit of negative charge. To this end, chlorides are widely defined as any material containing chlorine. Only one more. Chlorine Ion Formed.
From www.tessshebaylo.com
Salt Water Electrolysis Equation Tessshebaylo Chlorine Ion Formed For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +, and each chlorine atom in a. A chloride ion forms when a chlorine atom gains an electron. The formation of a chloride ion. Only one more electron is needed to achieve an octet in chlorine’s. Chlorine Ion Formed.
From chem.libretexts.org
4.3 The Reaction of Sodium with Chlorine Chemistry LibreTexts Chlorine Ion Formed The formation of a chloride ion. On the right, the chloride ion has 18 electrons and has a 1− charge. This fills the chlorine atom's outer shell, making it electronically stable. A chloride ion forms when a chlorine atom gains an electron. A neutral chlorine atom has seven electrons in its outermost shell. On the left, the chlorine atom has. Chlorine Ion Formed.
From wou.edu
CH150 Chapter 3 Ions and Ionic Compounds Chemistry Chlorine Ion Formed Only one more electron is needed to achieve an octet in chlorine’s valence shell. A chlorine atom (cl) gains one. Anion, which has one unit of negative charge. To this end, chlorides are widely defined as any material containing chlorine. On the left, the chlorine atom has 17 electrons. The formation of a chloride ion. On the right, the chloride. Chlorine Ion Formed.
From www.thesciencehive.co.uk
Bonding and Structure* — the science sauce Chlorine Ion Formed For example, when each sodium atom in a sample of sodium metal (group 1) gives up one electron to form a sodium cation, na +, and each chlorine atom in a. This fills the chlorine atom's outer shell, making it electronically stable. A chloride ion is a negatively charged ion i.e. A chlorine atom (cl) gains one. On the left,. Chlorine Ion Formed.