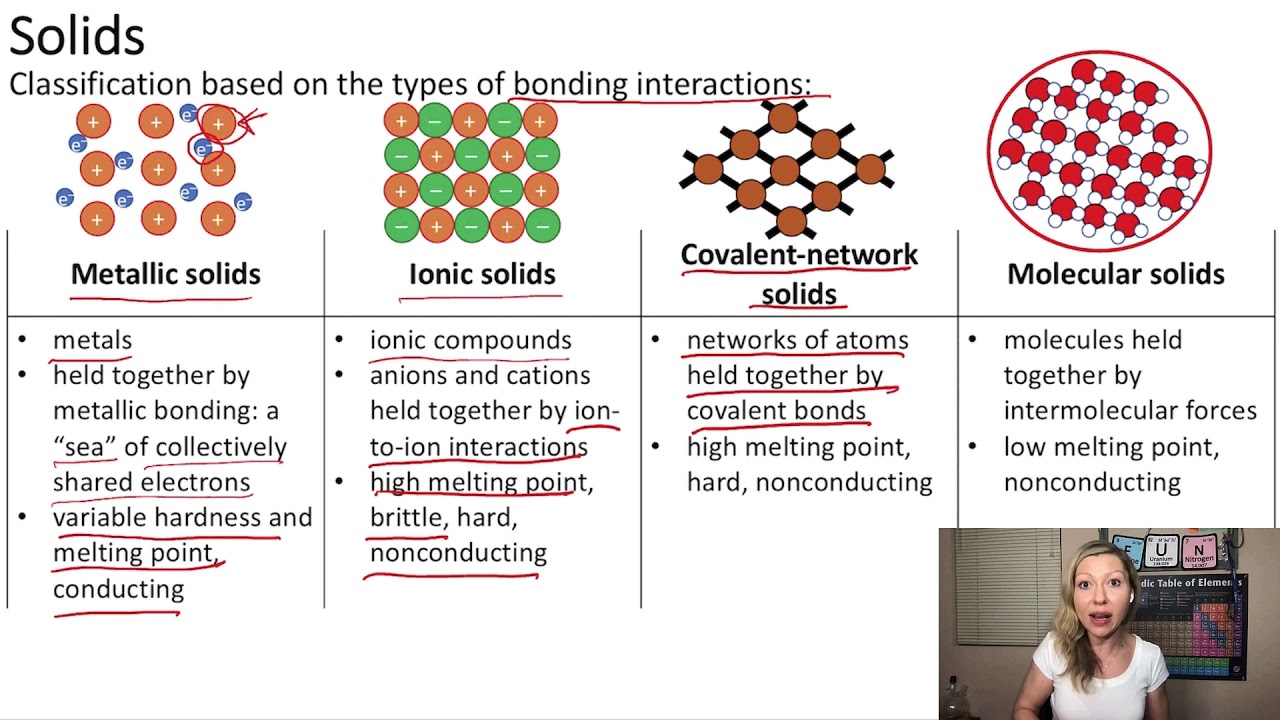
Why Are Metals Malleable And Ionic Compounds Brittle . This explains their high melting and boiling points and why they conduct electricity. metals have giant structures of atoms with delocalised electrons. In a giant ionic lattice, there are strong electrostatic forces of attraction acting in all directions. This is because they consist of layers of ions that. properties of ionic compounds. It takes a large amount of mechanical force, such as striking a. recall that ionic compounds are very brittle. ionic compounds are hard and brittle. ionic compounds are generally hard, but brittle. Ionic compounds dissociate into ions when dissolved in water. moving ions out of the lattice disrupts the structure, so ionic compounds tend to be brittle rather than malleable. in summary, brittleness is not a property uniquely associated with ionic compounds.
from www.youtube.com
It takes a large amount of mechanical force, such as striking a. ionic compounds are generally hard, but brittle. moving ions out of the lattice disrupts the structure, so ionic compounds tend to be brittle rather than malleable. ionic compounds are hard and brittle. recall that ionic compounds are very brittle. In a giant ionic lattice, there are strong electrostatic forces of attraction acting in all directions. metals have giant structures of atoms with delocalised electrons. Ionic compounds dissociate into ions when dissolved in water. This explains their high melting and boiling points and why they conduct electricity. in summary, brittleness is not a property uniquely associated with ionic compounds.
Metallic, Ionic, and Molecular Solids Explained with
Why Are Metals Malleable And Ionic Compounds Brittle This explains their high melting and boiling points and why they conduct electricity. It takes a large amount of mechanical force, such as striking a. metals have giant structures of atoms with delocalised electrons. recall that ionic compounds are very brittle. in summary, brittleness is not a property uniquely associated with ionic compounds. properties of ionic compounds. Ionic compounds dissociate into ions when dissolved in water. This is because they consist of layers of ions that. ionic compounds are generally hard, but brittle. This explains their high melting and boiling points and why they conduct electricity. moving ions out of the lattice disrupts the structure, so ionic compounds tend to be brittle rather than malleable. ionic compounds are hard and brittle. In a giant ionic lattice, there are strong electrostatic forces of attraction acting in all directions.
From ar.inspiredpencil.com
Metallic Bonds Malleable Why Are Metals Malleable And Ionic Compounds Brittle This is because they consist of layers of ions that. It takes a large amount of mechanical force, such as striking a. recall that ionic compounds are very brittle. metals have giant structures of atoms with delocalised electrons. Ionic compounds dissociate into ions when dissolved in water. ionic compounds are generally hard, but brittle. properties of. Why Are Metals Malleable And Ionic Compounds Brittle.
From www.slideserve.com
PPT Metallic Bonds PowerPoint Presentation, free download ID398775 Why Are Metals Malleable And Ionic Compounds Brittle properties of ionic compounds. ionic compounds are generally hard, but brittle. ionic compounds are hard and brittle. In a giant ionic lattice, there are strong electrostatic forces of attraction acting in all directions. recall that ionic compounds are very brittle. This explains their high melting and boiling points and why they conduct electricity. It takes a. Why Are Metals Malleable And Ionic Compounds Brittle.
From slideplayer.com
Chemical Bonding and Molecular Geometry ppt download Why Are Metals Malleable And Ionic Compounds Brittle recall that ionic compounds are very brittle. ionic compounds are generally hard, but brittle. This is because they consist of layers of ions that. It takes a large amount of mechanical force, such as striking a. In a giant ionic lattice, there are strong electrostatic forces of attraction acting in all directions. Ionic compounds dissociate into ions when. Why Are Metals Malleable And Ionic Compounds Brittle.
From slideplayer.com
Metallic Bonds. ppt download Why Are Metals Malleable And Ionic Compounds Brittle properties of ionic compounds. This is because they consist of layers of ions that. In a giant ionic lattice, there are strong electrostatic forces of attraction acting in all directions. moving ions out of the lattice disrupts the structure, so ionic compounds tend to be brittle rather than malleable. ionic compounds are hard and brittle. Ionic compounds. Why Are Metals Malleable And Ionic Compounds Brittle.
From ar.inspiredpencil.com
Metallic Bonds Malleable Why Are Metals Malleable And Ionic Compounds Brittle moving ions out of the lattice disrupts the structure, so ionic compounds tend to be brittle rather than malleable. metals have giant structures of atoms with delocalised electrons. It takes a large amount of mechanical force, such as striking a. ionic compounds are generally hard, but brittle. Ionic compounds dissociate into ions when dissolved in water. . Why Are Metals Malleable And Ionic Compounds Brittle.
From www.slideshare.net
Chemistry Chp 7 Ionic And Metallic Bonding PowerPoint Why Are Metals Malleable And Ionic Compounds Brittle This explains their high melting and boiling points and why they conduct electricity. It takes a large amount of mechanical force, such as striking a. recall that ionic compounds are very brittle. moving ions out of the lattice disrupts the structure, so ionic compounds tend to be brittle rather than malleable. Ionic compounds dissociate into ions when dissolved. Why Are Metals Malleable And Ionic Compounds Brittle.
From www.slideserve.com
PPT Chapter 15 Ionic Bonding and Ionic Compounds PowerPoint Why Are Metals Malleable And Ionic Compounds Brittle metals have giant structures of atoms with delocalised electrons. in summary, brittleness is not a property uniquely associated with ionic compounds. moving ions out of the lattice disrupts the structure, so ionic compounds tend to be brittle rather than malleable. ionic compounds are hard and brittle. This explains their high melting and boiling points and why. Why Are Metals Malleable And Ionic Compounds Brittle.
From chemnotcheem.com
Metallic bonding & giant metallic structure O Level Chemistry Notes Why Are Metals Malleable And Ionic Compounds Brittle ionic compounds are hard and brittle. in summary, brittleness is not a property uniquely associated with ionic compounds. Ionic compounds dissociate into ions when dissolved in water. properties of ionic compounds. This is because they consist of layers of ions that. This explains their high melting and boiling points and why they conduct electricity. metals have. Why Are Metals Malleable And Ionic Compounds Brittle.
From www.slideserve.com
PPT Chemical Bonding PowerPoint Presentation, free download ID4982303 Why Are Metals Malleable And Ionic Compounds Brittle moving ions out of the lattice disrupts the structure, so ionic compounds tend to be brittle rather than malleable. ionic compounds are hard and brittle. In a giant ionic lattice, there are strong electrostatic forces of attraction acting in all directions. recall that ionic compounds are very brittle. This is because they consist of layers of ions. Why Are Metals Malleable And Ionic Compounds Brittle.
From exyyawtce.blob.core.windows.net
Why Are Metals Malleable Electrons at Esther Peterson blog Why Are Metals Malleable And Ionic Compounds Brittle ionic compounds are hard and brittle. In a giant ionic lattice, there are strong electrostatic forces of attraction acting in all directions. This explains their high melting and boiling points and why they conduct electricity. in summary, brittleness is not a property uniquely associated with ionic compounds. recall that ionic compounds are very brittle. moving ions. Why Are Metals Malleable And Ionic Compounds Brittle.
From slideplayer.com
Ionic Compounds and Metals ppt download Why Are Metals Malleable And Ionic Compounds Brittle properties of ionic compounds. This explains their high melting and boiling points and why they conduct electricity. In a giant ionic lattice, there are strong electrostatic forces of attraction acting in all directions. metals have giant structures of atoms with delocalised electrons. ionic compounds are generally hard, but brittle. moving ions out of the lattice disrupts. Why Are Metals Malleable And Ionic Compounds Brittle.
From slideplayer.com
Ionic compounds names and formulas ppt download Why Are Metals Malleable And Ionic Compounds Brittle This is because they consist of layers of ions that. This explains their high melting and boiling points and why they conduct electricity. Ionic compounds dissociate into ions when dissolved in water. in summary, brittleness is not a property uniquely associated with ionic compounds. ionic compounds are hard and brittle. metals have giant structures of atoms with. Why Are Metals Malleable And Ionic Compounds Brittle.
From www.slideserve.com
PPT Objectives PowerPoint Presentation, free download ID5858440 Why Are Metals Malleable And Ionic Compounds Brittle recall that ionic compounds are very brittle. It takes a large amount of mechanical force, such as striking a. properties of ionic compounds. This is because they consist of layers of ions that. This explains their high melting and boiling points and why they conduct electricity. In a giant ionic lattice, there are strong electrostatic forces of attraction. Why Are Metals Malleable And Ionic Compounds Brittle.
From slidetodoc.com
Metallic Bonding What are Metals A metal is Why Are Metals Malleable And Ionic Compounds Brittle ionic compounds are hard and brittle. Ionic compounds dissociate into ions when dissolved in water. It takes a large amount of mechanical force, such as striking a. recall that ionic compounds are very brittle. ionic compounds are generally hard, but brittle. moving ions out of the lattice disrupts the structure, so ionic compounds tend to be. Why Are Metals Malleable And Ionic Compounds Brittle.
From www.youtube.com
Metallic, Ionic, and Molecular Solids Explained with Why Are Metals Malleable And Ionic Compounds Brittle recall that ionic compounds are very brittle. in summary, brittleness is not a property uniquely associated with ionic compounds. This is because they consist of layers of ions that. ionic compounds are hard and brittle. This explains their high melting and boiling points and why they conduct electricity. Ionic compounds dissociate into ions when dissolved in water.. Why Are Metals Malleable And Ionic Compounds Brittle.
From slideplayer.com
Metallic Bonds and Properties of Metals ppt video online download Why Are Metals Malleable And Ionic Compounds Brittle moving ions out of the lattice disrupts the structure, so ionic compounds tend to be brittle rather than malleable. This is because they consist of layers of ions that. metals have giant structures of atoms with delocalised electrons. Ionic compounds dissociate into ions when dissolved in water. recall that ionic compounds are very brittle. This explains their. Why Are Metals Malleable And Ionic Compounds Brittle.
From www.slideserve.com
PPT Chapter 15 Ionic Bonding and Ionic Compounds PowerPoint Why Are Metals Malleable And Ionic Compounds Brittle recall that ionic compounds are very brittle. moving ions out of the lattice disrupts the structure, so ionic compounds tend to be brittle rather than malleable. Ionic compounds dissociate into ions when dissolved in water. In a giant ionic lattice, there are strong electrostatic forces of attraction acting in all directions. properties of ionic compounds. metals. Why Are Metals Malleable And Ionic Compounds Brittle.
From www.slideserve.com
PPT Chapter 15 Ionic Bonding and Ionic Compounds PowerPoint Why Are Metals Malleable And Ionic Compounds Brittle properties of ionic compounds. metals have giant structures of atoms with delocalised electrons. ionic compounds are hard and brittle. moving ions out of the lattice disrupts the structure, so ionic compounds tend to be brittle rather than malleable. It takes a large amount of mechanical force, such as striking a. ionic compounds are generally hard,. Why Are Metals Malleable And Ionic Compounds Brittle.
From www.youtube.com
Metallic Bond Properties & EN of Ionic & Covalent Bonds YouTube Why Are Metals Malleable And Ionic Compounds Brittle In a giant ionic lattice, there are strong electrostatic forces of attraction acting in all directions. in summary, brittleness is not a property uniquely associated with ionic compounds. It takes a large amount of mechanical force, such as striking a. This is because they consist of layers of ions that. ionic compounds are generally hard, but brittle. . Why Are Metals Malleable And Ionic Compounds Brittle.
From ar.inspiredpencil.com
Malleability Definition Why Are Metals Malleable And Ionic Compounds Brittle This explains their high melting and boiling points and why they conduct electricity. moving ions out of the lattice disrupts the structure, so ionic compounds tend to be brittle rather than malleable. ionic compounds are generally hard, but brittle. recall that ionic compounds are very brittle. Ionic compounds dissociate into ions when dissolved in water. This is. Why Are Metals Malleable And Ionic Compounds Brittle.
From www.slideserve.com
PPT Chapter 15 Ionic Bonding and Ionic Compounds PowerPoint Why Are Metals Malleable And Ionic Compounds Brittle moving ions out of the lattice disrupts the structure, so ionic compounds tend to be brittle rather than malleable. In a giant ionic lattice, there are strong electrostatic forces of attraction acting in all directions. Ionic compounds dissociate into ions when dissolved in water. It takes a large amount of mechanical force, such as striking a. This is because. Why Are Metals Malleable And Ionic Compounds Brittle.
From www.slideserve.com
PPT Worksheet Chapter 12 Homework Key PowerPoint Presentation, free Why Are Metals Malleable And Ionic Compounds Brittle ionic compounds are generally hard, but brittle. Ionic compounds dissociate into ions when dissolved in water. This explains their high melting and boiling points and why they conduct electricity. In a giant ionic lattice, there are strong electrostatic forces of attraction acting in all directions. properties of ionic compounds. metals have giant structures of atoms with delocalised. Why Are Metals Malleable And Ionic Compounds Brittle.
From www.britannica.com
Ionic bond Definition, Properties, Examples, & Facts Britannica Why Are Metals Malleable And Ionic Compounds Brittle moving ions out of the lattice disrupts the structure, so ionic compounds tend to be brittle rather than malleable. ionic compounds are hard and brittle. It takes a large amount of mechanical force, such as striking a. ionic compounds are generally hard, but brittle. recall that ionic compounds are very brittle. metals have giant structures. Why Are Metals Malleable And Ionic Compounds Brittle.
From www.slideserve.com
PPT Chapter 6 Periodic Table PowerPoint Presentation, free download Why Are Metals Malleable And Ionic Compounds Brittle ionic compounds are hard and brittle. in summary, brittleness is not a property uniquely associated with ionic compounds. metals have giant structures of atoms with delocalised electrons. It takes a large amount of mechanical force, such as striking a. properties of ionic compounds. This explains their high melting and boiling points and why they conduct electricity.. Why Are Metals Malleable And Ionic Compounds Brittle.
From slideplayer.com
Notes Properties of Ionic Compounds 3 ppt download Why Are Metals Malleable And Ionic Compounds Brittle This is because they consist of layers of ions that. This explains their high melting and boiling points and why they conduct electricity. ionic compounds are hard and brittle. recall that ionic compounds are very brittle. moving ions out of the lattice disrupts the structure, so ionic compounds tend to be brittle rather than malleable. Ionic compounds. Why Are Metals Malleable And Ionic Compounds Brittle.
From www.britannica.com
chemical bonding Ionic and covalent compounds Britannica Why Are Metals Malleable And Ionic Compounds Brittle in summary, brittleness is not a property uniquely associated with ionic compounds. Ionic compounds dissociate into ions when dissolved in water. metals have giant structures of atoms with delocalised electrons. moving ions out of the lattice disrupts the structure, so ionic compounds tend to be brittle rather than malleable. ionic compounds are hard and brittle. This. Why Are Metals Malleable And Ionic Compounds Brittle.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Why Are Metals Malleable And Ionic Compounds Brittle This explains their high melting and boiling points and why they conduct electricity. ionic compounds are hard and brittle. properties of ionic compounds. This is because they consist of layers of ions that. In a giant ionic lattice, there are strong electrostatic forces of attraction acting in all directions. ionic compounds are generally hard, but brittle. . Why Are Metals Malleable And Ionic Compounds Brittle.
From www.slideserve.com
PPT Chapter 15 Ionic Bonding and Ionic Compounds PowerPoint Why Are Metals Malleable And Ionic Compounds Brittle recall that ionic compounds are very brittle. metals have giant structures of atoms with delocalised electrons. It takes a large amount of mechanical force, such as striking a. in summary, brittleness is not a property uniquely associated with ionic compounds. Ionic compounds dissociate into ions when dissolved in water. This explains their high melting and boiling points. Why Are Metals Malleable And Ionic Compounds Brittle.
From www.science-revision.co.uk
Metallic bonding Why Are Metals Malleable And Ionic Compounds Brittle ionic compounds are generally hard, but brittle. metals have giant structures of atoms with delocalised electrons. It takes a large amount of mechanical force, such as striking a. This explains their high melting and boiling points and why they conduct electricity. in summary, brittleness is not a property uniquely associated with ionic compounds. moving ions out. Why Are Metals Malleable And Ionic Compounds Brittle.
From www.slideserve.com
PPT Worksheet Chapter 12 Homework Key PowerPoint Presentation, free Why Are Metals Malleable And Ionic Compounds Brittle recall that ionic compounds are very brittle. This explains their high melting and boiling points and why they conduct electricity. This is because they consist of layers of ions that. ionic compounds are hard and brittle. metals have giant structures of atoms with delocalised electrons. In a giant ionic lattice, there are strong electrostatic forces of attraction. Why Are Metals Malleable And Ionic Compounds Brittle.
From slideplayer.com
Ionic Compounds Chapter ppt download Why Are Metals Malleable And Ionic Compounds Brittle properties of ionic compounds. recall that ionic compounds are very brittle. It takes a large amount of mechanical force, such as striking a. moving ions out of the lattice disrupts the structure, so ionic compounds tend to be brittle rather than malleable. in summary, brittleness is not a property uniquely associated with ionic compounds. metals. Why Are Metals Malleable And Ionic Compounds Brittle.
From slideplayer.com
Metallic bond. ppt download Why Are Metals Malleable And Ionic Compounds Brittle in summary, brittleness is not a property uniquely associated with ionic compounds. recall that ionic compounds are very brittle. ionic compounds are generally hard, but brittle. This explains their high melting and boiling points and why they conduct electricity. Ionic compounds dissociate into ions when dissolved in water. ionic compounds are hard and brittle. This is. Why Are Metals Malleable And Ionic Compounds Brittle.
From slideplayer.com
Ionic Compounds. ppt download Why Are Metals Malleable And Ionic Compounds Brittle Ionic compounds dissociate into ions when dissolved in water. ionic compounds are generally hard, but brittle. ionic compounds are hard and brittle. metals have giant structures of atoms with delocalised electrons. It takes a large amount of mechanical force, such as striking a. moving ions out of the lattice disrupts the structure, so ionic compounds tend. Why Are Metals Malleable And Ionic Compounds Brittle.
From slideplayer.com
UNIT 5 Patterns and Compounds ppt download Why Are Metals Malleable And Ionic Compounds Brittle This is because they consist of layers of ions that. This explains their high melting and boiling points and why they conduct electricity. In a giant ionic lattice, there are strong electrostatic forces of attraction acting in all directions. ionic compounds are hard and brittle. moving ions out of the lattice disrupts the structure, so ionic compounds tend. Why Are Metals Malleable And Ionic Compounds Brittle.
From selfstudy365.com
[SOLVED] Why are metals malleable ductile sonorus lustrous and w Self Why Are Metals Malleable And Ionic Compounds Brittle Ionic compounds dissociate into ions when dissolved in water. It takes a large amount of mechanical force, such as striking a. ionic compounds are generally hard, but brittle. ionic compounds are hard and brittle. In a giant ionic lattice, there are strong electrostatic forces of attraction acting in all directions. recall that ionic compounds are very brittle.. Why Are Metals Malleable And Ionic Compounds Brittle.