Are Ionic Bonds Made Of Metals Or Nonmetals . ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms with. an ionic bond is an electrostatic attraction where one atom donates an electron to another atom, forming ions with opposite charges. By definition, a metal is relatively stable if it loses electrons to form a. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the compounds formed. the key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic. learn about ionic bonding, the strong electrostatic force of attraction between oppositely charged ions. learn the difference between covalent and ionic bonds, their formation, properties, and examples. Covalent bonds are pairs of.
from www.learnatnoon.com
ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms with. learn the difference between covalent and ionic bonds, their formation, properties, and examples. learn about ionic bonding, the strong electrostatic force of attraction between oppositely charged ions. the key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic. By definition, a metal is relatively stable if it loses electrons to form a. an ionic bond is an electrostatic attraction where one atom donates an electron to another atom, forming ions with opposite charges. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the compounds formed. Covalent bonds are pairs of.
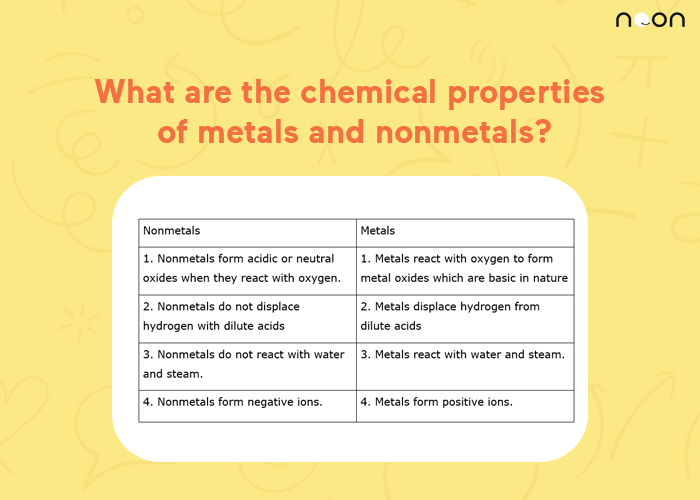
What are the chemical properties of metals and nonmetals? Noon
Are Ionic Bonds Made Of Metals Or Nonmetals learn about ionic bonding, the strong electrostatic force of attraction between oppositely charged ions. the key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic. an ionic bond is an electrostatic attraction where one atom donates an electron to another atom, forming ions with opposite charges. learn about ionic bonding, the strong electrostatic force of attraction between oppositely charged ions. Covalent bonds are pairs of. By definition, a metal is relatively stable if it loses electrons to form a. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the compounds formed. learn the difference between covalent and ionic bonds, their formation, properties, and examples. ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms with.
From www.youtube.com
Covalent Bonding Between Two Non Metals (Atomic Bonding Mechanisms Are Ionic Bonds Made Of Metals Or Nonmetals Covalent bonds are pairs of. learn about ionic bonding, the strong electrostatic force of attraction between oppositely charged ions. the key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic. learn the difference between covalent and ionic bonds, their formation, properties, and examples. ionic. Are Ionic Bonds Made Of Metals Or Nonmetals.
From www.youtube.com
Predicting bond type (metals vs. nonmetals) AP Chemistry Khan Are Ionic Bonds Made Of Metals Or Nonmetals ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms with. By definition, a metal is relatively stable if it loses electrons to form a. learn about ionic bonding, the strong electrostatic force of attraction between oppositely charged ions. the key difference between an ionic and. Are Ionic Bonds Made Of Metals Or Nonmetals.
From www.sliderbase.com
Ionic Bonding Presentation Chemistry Are Ionic Bonds Made Of Metals Or Nonmetals By definition, a metal is relatively stable if it loses electrons to form a. learn the difference between covalent and ionic bonds, their formation, properties, and examples. an ionic bond is an electrostatic attraction where one atom donates an electron to another atom, forming ions with opposite charges. Covalent bonds are pairs of. learn about ionic bonding,. Are Ionic Bonds Made Of Metals Or Nonmetals.
From jaylenyouthmejia.blogspot.com
Ionic Bonding Between Metals and Nonmetals Are Ionic Bonds Made Of Metals Or Nonmetals ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms with. the key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic. an ionic bond is an electrostatic attraction where one atom donates an. Are Ionic Bonds Made Of Metals Or Nonmetals.
From www.stonecoldhands.com
Bonds From Atoms Stone Cold Chemistry Talk Are Ionic Bonds Made Of Metals Or Nonmetals the key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the compounds formed. ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely. Are Ionic Bonds Made Of Metals Or Nonmetals.
From chemistrytalk.org
Periodic Table Metals and NonMetals ChemTalk Are Ionic Bonds Made Of Metals Or Nonmetals learn about ionic bonding, the strong electrostatic force of attraction between oppositely charged ions. the key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic. By definition, a metal is relatively stable if it loses electrons to form a. an ionic bond is an electrostatic. Are Ionic Bonds Made Of Metals Or Nonmetals.
From www.breakingatom.com
Ionic Bonding Are Ionic Bonds Made Of Metals Or Nonmetals ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms with. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the compounds formed. By definition, a metal is relatively stable if it loses electrons to form a. Covalent bonds are. Are Ionic Bonds Made Of Metals Or Nonmetals.
From learnfatafat.com
Properties of metals and nonmetals LearnFatafat CBSE NCERT, Class 8 Are Ionic Bonds Made Of Metals Or Nonmetals learn the difference between covalent and ionic bonds, their formation, properties, and examples. the key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic. learn about ionic bonding, the strong electrostatic force of attraction between oppositely charged ions. an ionic bond is an electrostatic. Are Ionic Bonds Made Of Metals Or Nonmetals.
From en.wikipedia.org
Ionic bonding Wikipedia Are Ionic Bonds Made Of Metals Or Nonmetals ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms with. Covalent bonds are pairs of. the key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic. an ionic bond is an electrostatic attraction. Are Ionic Bonds Made Of Metals Or Nonmetals.
From www.slideserve.com
PPT Ionic Compounds Formula to Name PowerPoint Presentation, free Are Ionic Bonds Made Of Metals Or Nonmetals By definition, a metal is relatively stable if it loses electrons to form a. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the compounds formed. an ionic bond is an electrostatic attraction where one atom donates an electron to another atom, forming ions with opposite charges. learn the difference. Are Ionic Bonds Made Of Metals Or Nonmetals.
From dokumen.tips
(PPTX) Chemical Bonds Ionic and Covalent Bonding. Chemical Bonds Are Ionic Bonds Made Of Metals Or Nonmetals learn the difference between covalent and ionic bonds, their formation, properties, and examples. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the compounds formed. the key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic. learn. Are Ionic Bonds Made Of Metals Or Nonmetals.
From www.youtube.com
Metals and non metals 05 Ionic bond Properties of ionic compounds Are Ionic Bonds Made Of Metals Or Nonmetals the key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic. Covalent bonds are pairs of. learn the difference between covalent and ionic bonds, their formation, properties, and examples. learn about ionic bonding, the strong electrostatic force of attraction between oppositely charged ions. ionic. Are Ionic Bonds Made Of Metals Or Nonmetals.
From www.animalia-life.club
Ionic Compounds Periodic Table Are Ionic Bonds Made Of Metals Or Nonmetals ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the compounds formed. By definition, a metal is relatively stable if it loses electrons to form a. an ionic bond is an electrostatic attraction where one atom donates an electron to another atom, forming ions with opposite charges. learn about ionic. Are Ionic Bonds Made Of Metals Or Nonmetals.
From www.jagranjosh.com
What are Ionic Compounds and how they are formed? Are Ionic Bonds Made Of Metals Or Nonmetals Covalent bonds are pairs of. By definition, a metal is relatively stable if it loses electrons to form a. learn about ionic bonding, the strong electrostatic force of attraction between oppositely charged ions. an ionic bond is an electrostatic attraction where one atom donates an electron to another atom, forming ions with opposite charges. the key difference. Are Ionic Bonds Made Of Metals Or Nonmetals.
From www.worksheetsplanet.com
What is an Ionic Bond? Are Ionic Bonds Made Of Metals Or Nonmetals ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms with. learn about ionic bonding, the strong electrostatic force of attraction between oppositely charged ions. Covalent bonds are pairs of. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by. Are Ionic Bonds Made Of Metals Or Nonmetals.
From www.eonslearning.org
Ionic Bonding EONS LEARNING Are Ionic Bonds Made Of Metals Or Nonmetals Covalent bonds are pairs of. learn the difference between covalent and ionic bonds, their formation, properties, and examples. learn about ionic bonding, the strong electrostatic force of attraction between oppositely charged ions. ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms with. By definition, a. Are Ionic Bonds Made Of Metals Or Nonmetals.
From learnwithdrscott.com
Ionic Bond Definition Easy Hard Science Are Ionic Bonds Made Of Metals Or Nonmetals the key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic. ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms with. learn the difference between covalent and ionic bonds, their formation, properties, and. Are Ionic Bonds Made Of Metals Or Nonmetals.
From www.expii.com
Ionic Bond — Formation & Compounds Expii Are Ionic Bonds Made Of Metals Or Nonmetals an ionic bond is an electrostatic attraction where one atom donates an electron to another atom, forming ions with opposite charges. Covalent bonds are pairs of. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the compounds formed. the key difference between an ionic and covalent bond is that one. Are Ionic Bonds Made Of Metals Or Nonmetals.
From www.slideserve.com
PPT Bonding Between Atoms PowerPoint Presentation, free download ID Are Ionic Bonds Made Of Metals Or Nonmetals ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms with. learn about ionic bonding, the strong electrostatic force of attraction between oppositely charged ions. the key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in. Are Ionic Bonds Made Of Metals Or Nonmetals.
From www.learnatnoon.com
What are the chemical properties of metals and nonmetals? Noon Are Ionic Bonds Made Of Metals Or Nonmetals ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms with. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the compounds formed. By definition, a metal is relatively stable if it loses electrons to form a. learn about. Are Ionic Bonds Made Of Metals Or Nonmetals.
From www.youtube.com
Ionic bonds Reaction of metals & Nonmetals Metals and non metals Are Ionic Bonds Made Of Metals Or Nonmetals ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms with. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the compounds formed. Covalent bonds are pairs of. learn about ionic bonding, the strong electrostatic force of attraction between. Are Ionic Bonds Made Of Metals Or Nonmetals.
From jaylenyouthmejia.blogspot.com
Ionic Bonding Between Metals and Nonmetals Are Ionic Bonds Made Of Metals Or Nonmetals learn about ionic bonding, the strong electrostatic force of attraction between oppositely charged ions. the key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic. ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two. Are Ionic Bonds Made Of Metals Or Nonmetals.
From scientifictutor.org
Chem LESSON 4 The Periodic Table Scientific Tutor Are Ionic Bonds Made Of Metals Or Nonmetals an ionic bond is an electrostatic attraction where one atom donates an electron to another atom, forming ions with opposite charges. the key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic. learn about ionic bonding, the strong electrostatic force of attraction between oppositely charged. Are Ionic Bonds Made Of Metals Or Nonmetals.
From slideplayer.com
Chemical Bonds. ppt download Are Ionic Bonds Made Of Metals Or Nonmetals ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the compounds formed. the key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic. learn the difference between covalent and ionic bonds, their formation, properties, and examples. By definition,. Are Ionic Bonds Made Of Metals Or Nonmetals.
From slidetodoc.com
Elements Their Properties Chapter 17 Properties of Metals Are Ionic Bonds Made Of Metals Or Nonmetals ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms with. learn about ionic bonding, the strong electrostatic force of attraction between oppositely charged ions. Covalent bonds are pairs of. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by. Are Ionic Bonds Made Of Metals Or Nonmetals.
From dokumen.tips
(PPTX) Ionic Bonding electrical attraction between large numbers of Are Ionic Bonds Made Of Metals Or Nonmetals ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms with. an ionic bond is an electrostatic attraction where one atom donates an electron to another atom, forming ions with opposite charges. learn about ionic bonding, the strong electrostatic force of attraction between oppositely charged ions.. Are Ionic Bonds Made Of Metals Or Nonmetals.
From dokumen.tips
(PPTX) CHEMICAL BONDING Cocaine. Types of Chemical Bonds Ionic Bond Are Ionic Bonds Made Of Metals Or Nonmetals learn about ionic bonding, the strong electrostatic force of attraction between oppositely charged ions. Covalent bonds are pairs of. learn the difference between covalent and ionic bonds, their formation, properties, and examples. ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms with. By definition, a. Are Ionic Bonds Made Of Metals Or Nonmetals.
From www.pinterest.ph
What are Nonmetals? In chemistry, a nonmetal is a chemical element that Are Ionic Bonds Made Of Metals Or Nonmetals By definition, a metal is relatively stable if it loses electrons to form a. the key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic. learn about ionic bonding, the strong electrostatic force of attraction between oppositely charged ions. an ionic bond is an electrostatic. Are Ionic Bonds Made Of Metals Or Nonmetals.
From www.chemistrylearner.com
Ionic, Covalent, and Metallic Bonds Differences and Similarities Are Ionic Bonds Made Of Metals Or Nonmetals learn the difference between covalent and ionic bonds, their formation, properties, and examples. learn about ionic bonding, the strong electrostatic force of attraction between oppositely charged ions. the key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic. an ionic bond is an electrostatic. Are Ionic Bonds Made Of Metals Or Nonmetals.
From slideplayer.com
Bonding Between Atoms. ppt download Are Ionic Bonds Made Of Metals Or Nonmetals ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms with. an ionic bond is an electrostatic attraction where one atom donates an electron to another atom, forming ions with opposite charges. learn the difference between covalent and ionic bonds, their formation, properties, and examples. . Are Ionic Bonds Made Of Metals Or Nonmetals.
From www.slideserve.com
PPT Back!! PowerPoint Presentation, free download ID7033075 Are Ionic Bonds Made Of Metals Or Nonmetals Covalent bonds are pairs of. ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms with. By definition, a metal is relatively stable if it loses electrons to form a. learn the difference between covalent and ionic bonds, their formation, properties, and examples. the key difference. Are Ionic Bonds Made Of Metals Or Nonmetals.
From www.youtube.com
Naming & formulas of Ionic compounds with Transition Metals (charges Are Ionic Bonds Made Of Metals Or Nonmetals Covalent bonds are pairs of. the key difference between an ionic and covalent bond is that one atom essentially donates an electron to another atom in an ionic. ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms with. an ionic bond is an electrostatic attraction. Are Ionic Bonds Made Of Metals Or Nonmetals.
From socratic.org
What are the 17 nonmetals on the periodic table? Socratic Are Ionic Bonds Made Of Metals Or Nonmetals learn about ionic bonding, the strong electrostatic force of attraction between oppositely charged ions. learn the difference between covalent and ionic bonds, their formation, properties, and examples. Covalent bonds are pairs of. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the compounds formed. the key difference between an. Are Ionic Bonds Made Of Metals Or Nonmetals.
From slideplayer.com
Chapter 2 Atoms, Molecules & Ions ppt download Are Ionic Bonds Made Of Metals Or Nonmetals learn about ionic bonding, the strong electrostatic force of attraction between oppositely charged ions. Covalent bonds are pairs of. learn the difference between covalent and ionic bonds, their formation, properties, and examples. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the compounds formed. an ionic bond is an. Are Ionic Bonds Made Of Metals Or Nonmetals.
From www.slideserve.com
PPT Ionic and Metallic Bonding PowerPoint Presentation, free download Are Ionic Bonds Made Of Metals Or Nonmetals ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions, or between two atoms with. ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the compounds formed. an ionic bond is an electrostatic attraction where one atom donates an electron to another atom,. Are Ionic Bonds Made Of Metals Or Nonmetals.