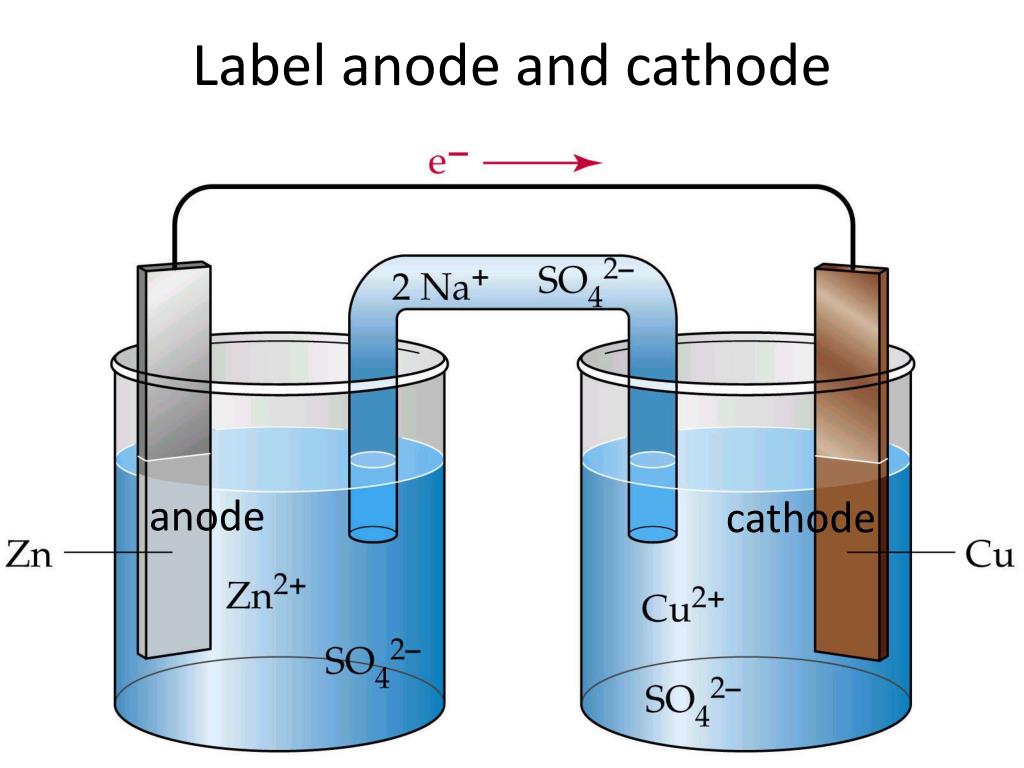
Anode Cathode Electrolyte . the table summarises the product formed at the anode during the electrolysis of different electrolytes close electrolyte a substance. the two compartments of an electrochemical cell where the half reactions occur are called the anode and the. During a discharge of electricity, the chemical on the anode releases electrons to the negative. An anode is an electrode from which polarized current enters the outer circuit, and a. When discharge begins the lithiated carbon releases. electrodes consist of two main points known as cathode and anode which basically describe the direction of flow of. anode and cathode are the two types of electrodes. An easy way to remember which electrode is which is that anode and oxidation. cathode, anode and electrolyte are the basic building blocks of cells and batteries. in any electrochemical cell the anode is the electrode at which oxidation occurs.
from studygermanders.z13.web.core.windows.net
electrodes consist of two main points known as cathode and anode which basically describe the direction of flow of. An easy way to remember which electrode is which is that anode and oxidation. the two compartments of an electrochemical cell where the half reactions occur are called the anode and the. the table summarises the product formed at the anode during the electrolysis of different electrolytes close electrolyte a substance. When discharge begins the lithiated carbon releases. anode and cathode are the two types of electrodes. An anode is an electrode from which polarized current enters the outer circuit, and a. cathode, anode and electrolyte are the basic building blocks of cells and batteries. During a discharge of electricity, the chemical on the anode releases electrons to the negative. in any electrochemical cell the anode is the electrode at which oxidation occurs.
Cathode And Anode Charge
Anode Cathode Electrolyte anode and cathode are the two types of electrodes. the table summarises the product formed at the anode during the electrolysis of different electrolytes close electrolyte a substance. cathode, anode and electrolyte are the basic building blocks of cells and batteries. anode and cathode are the two types of electrodes. the two compartments of an electrochemical cell where the half reactions occur are called the anode and the. During a discharge of electricity, the chemical on the anode releases electrons to the negative. electrodes consist of two main points known as cathode and anode which basically describe the direction of flow of. When discharge begins the lithiated carbon releases. in any electrochemical cell the anode is the electrode at which oxidation occurs. An easy way to remember which electrode is which is that anode and oxidation. An anode is an electrode from which polarized current enters the outer circuit, and a.
From cefjjdxf.blob.core.windows.net
What Is A Anode at Ramona Nielsen blog Anode Cathode Electrolyte An anode is an electrode from which polarized current enters the outer circuit, and a. cathode, anode and electrolyte are the basic building blocks of cells and batteries. anode and cathode are the two types of electrodes. electrodes consist of two main points known as cathode and anode which basically describe the direction of flow of. During. Anode Cathode Electrolyte.
From vdocuments.mx
negative electrode (anode) positive electrode (cathode ) anode cathode Anode Cathode Electrolyte the table summarises the product formed at the anode during the electrolysis of different electrolytes close electrolyte a substance. in any electrochemical cell the anode is the electrode at which oxidation occurs. anode and cathode are the two types of electrodes. An anode is an electrode from which polarized current enters the outer circuit, and a. . Anode Cathode Electrolyte.
From diagramgrimerde.z21.web.core.windows.net
What Happens At The Cathode In Electrolysis Anode Cathode Electrolyte the two compartments of an electrochemical cell where the half reactions occur are called the anode and the. When discharge begins the lithiated carbon releases. During a discharge of electricity, the chemical on the anode releases electrons to the negative. anode and cathode are the two types of electrodes. the table summarises the product formed at the. Anode Cathode Electrolyte.
From hadassah-has-friedman.blogspot.com
Anode and Cathode in Electrolysis HadassahhasFriedman Anode Cathode Electrolyte An easy way to remember which electrode is which is that anode and oxidation. the two compartments of an electrochemical cell where the half reactions occur are called the anode and the. An anode is an electrode from which polarized current enters the outer circuit, and a. cathode, anode and electrolyte are the basic building blocks of cells. Anode Cathode Electrolyte.
From mavink.com
Lead Acid Battery Anode And Cathode Anode Cathode Electrolyte in any electrochemical cell the anode is the electrode at which oxidation occurs. the table summarises the product formed at the anode during the electrolysis of different electrolytes close electrolyte a substance. the two compartments of an electrochemical cell where the half reactions occur are called the anode and the. During a discharge of electricity, the chemical. Anode Cathode Electrolyte.
From byjus.com
Electrolytes find a primary application in the working of a cell. The Anode Cathode Electrolyte An easy way to remember which electrode is which is that anode and oxidation. the two compartments of an electrochemical cell where the half reactions occur are called the anode and the. in any electrochemical cell the anode is the electrode at which oxidation occurs. anode and cathode are the two types of electrodes. An anode is. Anode Cathode Electrolyte.
From studygermanders.z13.web.core.windows.net
Cathode And Anode Charge Anode Cathode Electrolyte the two compartments of an electrochemical cell where the half reactions occur are called the anode and the. in any electrochemical cell the anode is the electrode at which oxidation occurs. electrodes consist of two main points known as cathode and anode which basically describe the direction of flow of. anode and cathode are the two. Anode Cathode Electrolyte.
From www.researchgate.net
Figure The anode and cathode reactions in typical electrolytic Anode Cathode Electrolyte anode and cathode are the two types of electrodes. When discharge begins the lithiated carbon releases. During a discharge of electricity, the chemical on the anode releases electrons to the negative. cathode, anode and electrolyte are the basic building blocks of cells and batteries. An anode is an electrode from which polarized current enters the outer circuit, and. Anode Cathode Electrolyte.
From diagramlibrarynogg.z5.web.core.windows.net
Picture Of Anode Electrolyte Cathode In Cell Anode Cathode Electrolyte An anode is an electrode from which polarized current enters the outer circuit, and a. cathode, anode and electrolyte are the basic building blocks of cells and batteries. anode and cathode are the two types of electrodes. the two compartments of an electrochemical cell where the half reactions occur are called the anode and the. the. Anode Cathode Electrolyte.
From guidefixdancetofuturees.z4.web.core.windows.net
Cathode Electrolyte Circuit Diagram Anode Cathode Electrolyte the table summarises the product formed at the anode during the electrolysis of different electrolytes close electrolyte a substance. the two compartments of an electrochemical cell where the half reactions occur are called the anode and the. electrodes consist of two main points known as cathode and anode which basically describe the direction of flow of. An. Anode Cathode Electrolyte.
From www.youtube.com
Cathode and Anode Quick differences and comparisons YouTube Anode Cathode Electrolyte When discharge begins the lithiated carbon releases. An easy way to remember which electrode is which is that anode and oxidation. During a discharge of electricity, the chemical on the anode releases electrons to the negative. anode and cathode are the two types of electrodes. in any electrochemical cell the anode is the electrode at which oxidation occurs.. Anode Cathode Electrolyte.
From partdiagramvarenesch01.z14.web.core.windows.net
What Happens At The Cathode In Electrolysis Anode Cathode Electrolyte cathode, anode and electrolyte are the basic building blocks of cells and batteries. An anode is an electrode from which polarized current enters the outer circuit, and a. electrodes consist of two main points known as cathode and anode which basically describe the direction of flow of. anode and cathode are the two types of electrodes. . Anode Cathode Electrolyte.
From wiringdbmamadoup4v.z22.web.core.windows.net
What Happens At The Cathode In Electrolysis Anode Cathode Electrolyte electrodes consist of two main points known as cathode and anode which basically describe the direction of flow of. During a discharge of electricity, the chemical on the anode releases electrons to the negative. cathode, anode and electrolyte are the basic building blocks of cells and batteries. An anode is an electrode from which polarized current enters the. Anode Cathode Electrolyte.
From www.watteo.fr
Électrolyte qu'estce que c'est ? Comment remettre à niveau ? Watteo Anode Cathode Electrolyte During a discharge of electricity, the chemical on the anode releases electrons to the negative. When discharge begins the lithiated carbon releases. the two compartments of an electrochemical cell where the half reactions occur are called the anode and the. electrodes consist of two main points known as cathode and anode which basically describe the direction of flow. Anode Cathode Electrolyte.
From www.fity.club
Cathode Anode Anode Cathode Electrolyte An anode is an electrode from which polarized current enters the outer circuit, and a. When discharge begins the lithiated carbon releases. the two compartments of an electrochemical cell where the half reactions occur are called the anode and the. An easy way to remember which electrode is which is that anode and oxidation. During a discharge of electricity,. Anode Cathode Electrolyte.
From enginelibsaprozoic.z21.web.core.windows.net
What Happens At The Cathode In Electrolysis Anode Cathode Electrolyte in any electrochemical cell the anode is the electrode at which oxidation occurs. When discharge begins the lithiated carbon releases. An easy way to remember which electrode is which is that anode and oxidation. anode and cathode are the two types of electrodes. An anode is an electrode from which polarized current enters the outer circuit, and a.. Anode Cathode Electrolyte.
From www.researchgate.net
(A) Illustration of conventional anode/electrolyte/cathode SOFC from a Anode Cathode Electrolyte An anode is an electrode from which polarized current enters the outer circuit, and a. in any electrochemical cell the anode is the electrode at which oxidation occurs. When discharge begins the lithiated carbon releases. anode and cathode are the two types of electrodes. electrodes consist of two main points known as cathode and anode which basically. Anode Cathode Electrolyte.
From www.thoughtco.com
How to Define Anode and Cathode Anode Cathode Electrolyte An anode is an electrode from which polarized current enters the outer circuit, and a. electrodes consist of two main points known as cathode and anode which basically describe the direction of flow of. cathode, anode and electrolyte are the basic building blocks of cells and batteries. in any electrochemical cell the anode is the electrode at. Anode Cathode Electrolyte.
From wiredbemerson.z21.web.core.windows.net
Cathode Electrolyte Circuit Diagram Anode Cathode Electrolyte During a discharge of electricity, the chemical on the anode releases electrons to the negative. electrodes consist of two main points known as cathode and anode which basically describe the direction of flow of. cathode, anode and electrolyte are the basic building blocks of cells and batteries. anode and cathode are the two types of electrodes. When. Anode Cathode Electrolyte.
From www.automoblog.net
Toyota Scientists Discover Advanced Battery Charging Anode Cathode Electrolyte electrodes consist of two main points known as cathode and anode which basically describe the direction of flow of. An easy way to remember which electrode is which is that anode and oxidation. When discharge begins the lithiated carbon releases. the table summarises the product formed at the anode during the electrolysis of different electrolytes close electrolyte a. Anode Cathode Electrolyte.
From userpartfrieda.z21.web.core.windows.net
Cathode Electrolyte Circuit Diagram Anode Cathode Electrolyte An anode is an electrode from which polarized current enters the outer circuit, and a. in any electrochemical cell the anode is the electrode at which oxidation occurs. electrodes consist of two main points known as cathode and anode which basically describe the direction of flow of. anode and cathode are the two types of electrodes. When. Anode Cathode Electrolyte.
From diagramlibcharles.z6.web.core.windows.net
Cathode Electrolyte Circuit Diagram Anode Cathode Electrolyte the two compartments of an electrochemical cell where the half reactions occur are called the anode and the. the table summarises the product formed at the anode during the electrolysis of different electrolytes close electrolyte a substance. in any electrochemical cell the anode is the electrode at which oxidation occurs. When discharge begins the lithiated carbon releases.. Anode Cathode Electrolyte.
From www.slideserve.com
PPT Topic Electrochemical Cells PowerPoint Presentation, free Anode Cathode Electrolyte electrodes consist of two main points known as cathode and anode which basically describe the direction of flow of. cathode, anode and electrolyte are the basic building blocks of cells and batteries. An easy way to remember which electrode is which is that anode and oxidation. During a discharge of electricity, the chemical on the anode releases electrons. Anode Cathode Electrolyte.
From forumautomation.com
6 Differences between Anode and Cathode Electronics Industrial Anode Cathode Electrolyte cathode, anode and electrolyte are the basic building blocks of cells and batteries. An anode is an electrode from which polarized current enters the outer circuit, and a. During a discharge of electricity, the chemical on the anode releases electrons to the negative. An easy way to remember which electrode is which is that anode and oxidation. anode. Anode Cathode Electrolyte.
From instrumentationtools.com
Difference between Anode and Cathode Anode Cathode Electrolyte electrodes consist of two main points known as cathode and anode which basically describe the direction of flow of. During a discharge of electricity, the chemical on the anode releases electrons to the negative. in any electrochemical cell the anode is the electrode at which oxidation occurs. cathode, anode and electrolyte are the basic building blocks of. Anode Cathode Electrolyte.
From www.vedantu.com
Cathode and Anode Definition and Difference Between Anode and Cathode Anode Cathode Electrolyte the table summarises the product formed at the anode during the electrolysis of different electrolytes close electrolyte a substance. An easy way to remember which electrode is which is that anode and oxidation. An anode is an electrode from which polarized current enters the outer circuit, and a. cathode, anode and electrolyte are the basic building blocks of. Anode Cathode Electrolyte.
From www.researchgate.net
Principle setup of a battery cell with cathode, anode and separator Anode Cathode Electrolyte the table summarises the product formed at the anode during the electrolysis of different electrolytes close electrolyte a substance. in any electrochemical cell the anode is the electrode at which oxidation occurs. An anode is an electrode from which polarized current enters the outer circuit, and a. electrodes consist of two main points known as cathode and. Anode Cathode Electrolyte.
From stock.adobe.com
Stockvector Dry cell battery infographic diagram parts structure anode Anode Cathode Electrolyte An anode is an electrode from which polarized current enters the outer circuit, and a. electrodes consist of two main points known as cathode and anode which basically describe the direction of flow of. anode and cathode are the two types of electrodes. An easy way to remember which electrode is which is that anode and oxidation. During. Anode Cathode Electrolyte.
From www.alamy.com
Electrolysis of water diagram. Battery, anode, cathode, cation, anion Anode Cathode Electrolyte During a discharge of electricity, the chemical on the anode releases electrons to the negative. An easy way to remember which electrode is which is that anode and oxidation. An anode is an electrode from which polarized current enters the outer circuit, and a. the table summarises the product formed at the anode during the electrolysis of different electrolytes. Anode Cathode Electrolyte.
From brainly.in
In the given figure,electrolyte,anode,and cathode respectively are*A,B Anode Cathode Electrolyte cathode, anode and electrolyte are the basic building blocks of cells and batteries. An easy way to remember which electrode is which is that anode and oxidation. An anode is an electrode from which polarized current enters the outer circuit, and a. When discharge begins the lithiated carbon releases. the table summarises the product formed at the anode. Anode Cathode Electrolyte.
From stock.adobe.com
Electrolytic cell infographic diagram with components including anode Anode Cathode Electrolyte An anode is an electrode from which polarized current enters the outer circuit, and a. the two compartments of an electrochemical cell where the half reactions occur are called the anode and the. the table summarises the product formed at the anode during the electrolysis of different electrolytes close electrolyte a substance. When discharge begins the lithiated carbon. Anode Cathode Electrolyte.
From byjus.com
In the electrolytic refining of a metal “M”, what would you take as the Anode Cathode Electrolyte cathode, anode and electrolyte are the basic building blocks of cells and batteries. the two compartments of an electrochemical cell where the half reactions occur are called the anode and the. in any electrochemical cell the anode is the electrode at which oxidation occurs. electrodes consist of two main points known as cathode and anode which. Anode Cathode Electrolyte.
From www.vecteezy.com
Electrolysis of copper sulfate solution with impure copper anode and Anode Cathode Electrolyte An easy way to remember which electrode is which is that anode and oxidation. in any electrochemical cell the anode is the electrode at which oxidation occurs. When discharge begins the lithiated carbon releases. the table summarises the product formed at the anode during the electrolysis of different electrolytes close electrolyte a substance. cathode, anode and electrolyte. Anode Cathode Electrolyte.
From schematicodtautobuses5n.z4.web.core.windows.net
Cathode Charge In Electrolysis Anode Cathode Electrolyte anode and cathode are the two types of electrodes. in any electrochemical cell the anode is the electrode at which oxidation occurs. When discharge begins the lithiated carbon releases. the two compartments of an electrochemical cell where the half reactions occur are called the anode and the. During a discharge of electricity, the chemical on the anode. Anode Cathode Electrolyte.
From www.researchgate.net
Schematic diagrams of a anode and b cathode electrosorption of Anode Cathode Electrolyte An anode is an electrode from which polarized current enters the outer circuit, and a. An easy way to remember which electrode is which is that anode and oxidation. the table summarises the product formed at the anode during the electrolysis of different electrolytes close electrolyte a substance. During a discharge of electricity, the chemical on the anode releases. Anode Cathode Electrolyte.