Medical Device Classification In Japan . Jmdn is a system of classifying and identifying medical devices based on gmdn (global medical device nomenclature). Find out how to obtain. Medical devices in japan are classified based on risk to the human body. How medical devices are classified in japan. The mhlw uses a tiered classification system for. Approval requirements for medical devices vary by. Medical device classification and regulation. This web page provides information about the standards for medical devices in japan, such as certification criteria, nomenclature, and review. Learn about the regulatory authorities, classification, procedures and criteria for medical devices in japan. Mds are classified into 4 categories (class i to iv) according to risk level. Medical devices, in japan, are classified into the following four categories based on their intended use and safety risk.
from www.mi-3.co.uk
Medical devices in japan are classified based on risk to the human body. Jmdn is a system of classifying and identifying medical devices based on gmdn (global medical device nomenclature). Mds are classified into 4 categories (class i to iv) according to risk level. Find out how to obtain. Approval requirements for medical devices vary by. Learn about the regulatory authorities, classification, procedures and criteria for medical devices in japan. This web page provides information about the standards for medical devices in japan, such as certification criteria, nomenclature, and review. Medical devices, in japan, are classified into the following four categories based on their intended use and safety risk. How medical devices are classified in japan. Medical device classification and regulation.
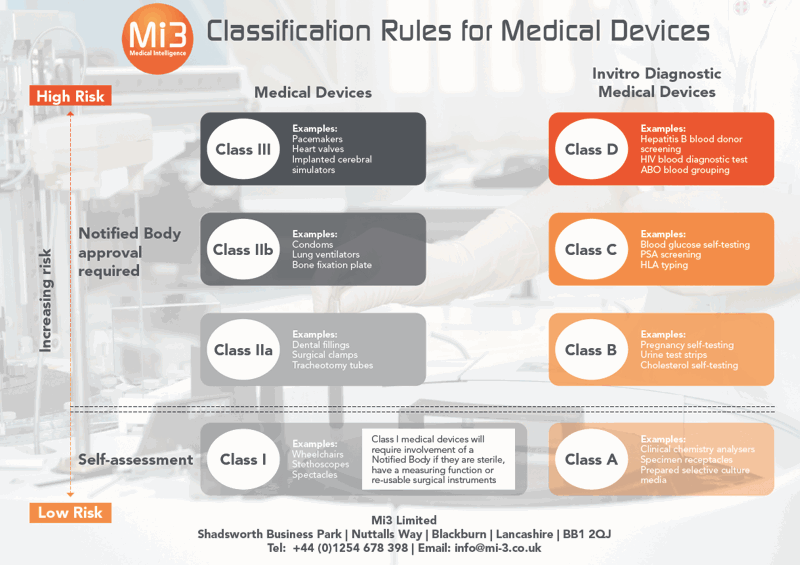
Your free guide to current MDR Classification Rules Mi3
Medical Device Classification In Japan Medical device classification and regulation. Find out how to obtain. Mds are classified into 4 categories (class i to iv) according to risk level. This web page provides information about the standards for medical devices in japan, such as certification criteria, nomenclature, and review. Learn about the regulatory authorities, classification, procedures and criteria for medical devices in japan. The mhlw uses a tiered classification system for. How medical devices are classified in japan. Approval requirements for medical devices vary by. Jmdn is a system of classifying and identifying medical devices based on gmdn (global medical device nomenclature). Medical device classification and regulation. Medical devices in japan are classified based on risk to the human body. Medical devices, in japan, are classified into the following four categories based on their intended use and safety risk.
From www.mi-3.co.uk
Your free guide to current MDR Classification Rules Mi3 Medical Device Classification In Japan The mhlw uses a tiered classification system for. Medical devices in japan are classified based on risk to the human body. Mds are classified into 4 categories (class i to iv) according to risk level. Medical device classification and regulation. Medical devices, in japan, are classified into the following four categories based on their intended use and safety risk. Find. Medical Device Classification In Japan.
From credevo.com
Top6 Considerations To Register Medical device In Japan Credevo Articles Medical Device Classification In Japan The mhlw uses a tiered classification system for. Medical devices in japan are classified based on risk to the human body. Learn about the regulatory authorities, classification, procedures and criteria for medical devices in japan. Medical device classification and regulation. Find out how to obtain. Approval requirements for medical devices vary by. Jmdn is a system of classifying and identifying. Medical Device Classification In Japan.
From japanhpn.org
Japan Health Policy NOW 6.4 Medical Devices Medical Device Classification In Japan How medical devices are classified in japan. Mds are classified into 4 categories (class i to iv) according to risk level. Jmdn is a system of classifying and identifying medical devices based on gmdn (global medical device nomenclature). Medical device classification and regulation. Find out how to obtain. Learn about the regulatory authorities, classification, procedures and criteria for medical devices. Medical Device Classification In Japan.
From www.slideshare.net
Japan Medical Device Regulatory Updates and Recent Revisions Medical Device Classification In Japan The mhlw uses a tiered classification system for. Mds are classified into 4 categories (class i to iv) according to risk level. This web page provides information about the standards for medical devices in japan, such as certification criteria, nomenclature, and review. Jmdn is a system of classifying and identifying medical devices based on gmdn (global medical device nomenclature). Medical. Medical Device Classification In Japan.
From www.sycaitechnologies.com
Overview on the regulatory path for software medical devices Medical Device Classification In Japan Jmdn is a system of classifying and identifying medical devices based on gmdn (global medical device nomenclature). The mhlw uses a tiered classification system for. This web page provides information about the standards for medical devices in japan, such as certification criteria, nomenclature, and review. Mds are classified into 4 categories (class i to iv) according to risk level. Approval. Medical Device Classification In Japan.
From www.freyrsolutions.com
Factors Determining Medical Device Registration in Japan Freyr Medical Device Classification In Japan Approval requirements for medical devices vary by. Medical devices in japan are classified based on risk to the human body. Jmdn is a system of classifying and identifying medical devices based on gmdn (global medical device nomenclature). Medical devices, in japan, are classified into the following four categories based on their intended use and safety risk. This web page provides. Medical Device Classification In Japan.
From apacmed.org
Medical Device Regulation Importance and Examples in APAC Medical Device Classification In Japan Medical device classification and regulation. Medical devices in japan are classified based on risk to the human body. Medical devices, in japan, are classified into the following four categories based on their intended use and safety risk. Approval requirements for medical devices vary by. Learn about the regulatory authorities, classification, procedures and criteria for medical devices in japan. Mds are. Medical Device Classification In Japan.
From credevo.com
Japan Medical Device Approval Process Credevo Articles Medical Device Classification In Japan Mds are classified into 4 categories (class i to iv) according to risk level. Medical device classification and regulation. Jmdn is a system of classifying and identifying medical devices based on gmdn (global medical device nomenclature). This web page provides information about the standards for medical devices in japan, such as certification criteria, nomenclature, and review. Find out how to. Medical Device Classification In Japan.
From www.emergobyul.com
Japanese Regulatory Approval Process for Medical Devices Emergo by UL Medical Device Classification In Japan This web page provides information about the standards for medical devices in japan, such as certification criteria, nomenclature, and review. Mds are classified into 4 categories (class i to iv) according to risk level. Medical devices in japan are classified based on risk to the human body. How medical devices are classified in japan. Find out how to obtain. Approval. Medical Device Classification In Japan.
From english.nmpa.gov.cn
Rules for Classification of Medical Devices Medical Device Classification In Japan Jmdn is a system of classifying and identifying medical devices based on gmdn (global medical device nomenclature). This web page provides information about the standards for medical devices in japan, such as certification criteria, nomenclature, and review. Approval requirements for medical devices vary by. Medical devices in japan are classified based on risk to the human body. How medical devices. Medical Device Classification In Japan.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Medical Device Classification In Japan Medical devices, in japan, are classified into the following four categories based on their intended use and safety risk. Jmdn is a system of classifying and identifying medical devices based on gmdn (global medical device nomenclature). Medical devices in japan are classified based on risk to the human body. How medical devices are classified in japan. Learn about the regulatory. Medical Device Classification In Japan.
From www.researchgate.net
Medical Device Classification a Download Scientific Diagram Medical Device Classification In Japan Mds are classified into 4 categories (class i to iv) according to risk level. Medical devices, in japan, are classified into the following four categories based on their intended use and safety risk. Medical devices in japan are classified based on risk to the human body. This web page provides information about the standards for medical devices in japan, such. Medical Device Classification In Japan.
From www.researchgate.net
5.3 Overview on the classification of medical devices and the Medical Device Classification In Japan How medical devices are classified in japan. The mhlw uses a tiered classification system for. Medical devices, in japan, are classified into the following four categories based on their intended use and safety risk. Approval requirements for medical devices vary by. Learn about the regulatory authorities, classification, procedures and criteria for medical devices in japan. Find out how to obtain.. Medical Device Classification In Japan.
From news.iscas.co
Interoperability standards for medical device integration in the OR and Medical Device Classification In Japan Find out how to obtain. Approval requirements for medical devices vary by. Learn about the regulatory authorities, classification, procedures and criteria for medical devices in japan. Mds are classified into 4 categories (class i to iv) according to risk level. Medical devices, in japan, are classified into the following four categories based on their intended use and safety risk. Medical. Medical Device Classification In Japan.
From gamma.app
Understanding Medical Device Classification in Japan Medical Device Classification In Japan Medical devices, in japan, are classified into the following four categories based on their intended use and safety risk. Find out how to obtain. Mds are classified into 4 categories (class i to iv) according to risk level. Learn about the regulatory authorities, classification, procedures and criteria for medical devices in japan. This web page provides information about the standards. Medical Device Classification In Japan.
From mungfali.com
Classification Of Medical Devices Medical Device Classification In Japan How medical devices are classified in japan. This web page provides information about the standards for medical devices in japan, such as certification criteria, nomenclature, and review. Learn about the regulatory authorities, classification, procedures and criteria for medical devices in japan. The mhlw uses a tiered classification system for. Approval requirements for medical devices vary by. Mds are classified into. Medical Device Classification In Japan.
From www.regdesk.co
An Overview of Medical Device Regulations in Japan RegDesk Medical Device Classification In Japan This web page provides information about the standards for medical devices in japan, such as certification criteria, nomenclature, and review. Medical device classification and regulation. Learn about the regulatory authorities, classification, procedures and criteria for medical devices in japan. Medical devices in japan are classified based on risk to the human body. Mds are classified into 4 categories (class i. Medical Device Classification In Japan.
From ciqa.net
How to Determine the Medical Device Classification? • Follow us for more. Medical Device Classification In Japan Mds are classified into 4 categories (class i to iv) according to risk level. Jmdn is a system of classifying and identifying medical devices based on gmdn (global medical device nomenclature). This web page provides information about the standards for medical devices in japan, such as certification criteria, nomenclature, and review. How medical devices are classified in japan. Learn about. Medical Device Classification In Japan.
From www.researchgate.net
Comparison of marketing authorization procedure of medical device in Medical Device Classification In Japan Medical device classification and regulation. Approval requirements for medical devices vary by. Medical devices in japan are classified based on risk to the human body. Jmdn is a system of classifying and identifying medical devices based on gmdn (global medical device nomenclature). Find out how to obtain. This web page provides information about the standards for medical devices in japan,. Medical Device Classification In Japan.
From www.slideshare.net
Japan Medical Devices Market Medical Device Classification In Japan Approval requirements for medical devices vary by. The mhlw uses a tiered classification system for. Medical devices in japan are classified based on risk to the human body. Medical device classification and regulation. Medical devices, in japan, are classified into the following four categories based on their intended use and safety risk. How medical devices are classified in japan. Jmdn. Medical Device Classification In Japan.
From www.scas.co.jp
Development/Application Support Service for Medical Devices Medical Device Classification In Japan Jmdn is a system of classifying and identifying medical devices based on gmdn (global medical device nomenclature). Medical devices in japan are classified based on risk to the human body. Mds are classified into 4 categories (class i to iv) according to risk level. Approval requirements for medical devices vary by. How medical devices are classified in japan. Medical devices,. Medical Device Classification In Japan.
From www.emergobyul.com
Medical Device and IVD Classification in Japan Emergo by UL Medical Device Classification In Japan How medical devices are classified in japan. Medical devices, in japan, are classified into the following four categories based on their intended use and safety risk. The mhlw uses a tiered classification system for. Find out how to obtain. This web page provides information about the standards for medical devices in japan, such as certification criteria, nomenclature, and review. Medical. Medical Device Classification In Japan.
From www.slideshare.net
Japan medical device approval chart Emergo Group Medical Device Classification In Japan Medical device classification and regulation. Jmdn is a system of classifying and identifying medical devices based on gmdn (global medical device nomenclature). Medical devices, in japan, are classified into the following four categories based on their intended use and safety risk. Medical devices in japan are classified based on risk to the human body. This web page provides information about. Medical Device Classification In Japan.
From www.greenlight.guru
Medical Device Classifications Determine Your Device Class Medical Device Classification In Japan The mhlw uses a tiered classification system for. Find out how to obtain. Medical device classification and regulation. This web page provides information about the standards for medical devices in japan, such as certification criteria, nomenclature, and review. Mds are classified into 4 categories (class i to iv) according to risk level. How medical devices are classified in japan. Medical. Medical Device Classification In Japan.
From www.youtube.com
Japan Medical Device Registration Chapter 1 Overview YouTube Medical Device Classification In Japan Approval requirements for medical devices vary by. The mhlw uses a tiered classification system for. Mds are classified into 4 categories (class i to iv) according to risk level. Find out how to obtain. Learn about the regulatory authorities, classification, procedures and criteria for medical devices in japan. Medical devices in japan are classified based on risk to the human. Medical Device Classification In Japan.
From www.youtube.com
Japan Medical Device Registration Chapter 2 Classification YouTube Medical Device Classification In Japan Mds are classified into 4 categories (class i to iv) according to risk level. This web page provides information about the standards for medical devices in japan, such as certification criteria, nomenclature, and review. Medical devices in japan are classified based on risk to the human body. Find out how to obtain. Medical devices, in japan, are classified into the. Medical Device Classification In Japan.
From credevo.com
Japan Medical Device Approval Process Credevo Articles Medical Device Classification In Japan Jmdn is a system of classifying and identifying medical devices based on gmdn (global medical device nomenclature). How medical devices are classified in japan. Find out how to obtain. Mds are classified into 4 categories (class i to iv) according to risk level. Medical devices, in japan, are classified into the following four categories based on their intended use and. Medical Device Classification In Japan.
From www.greenlight.guru
Medical Device Classification Guide How To Determine Your Device Class Medical Device Classification In Japan Mds are classified into 4 categories (class i to iv) according to risk level. Find out how to obtain. Medical device classification and regulation. The mhlw uses a tiered classification system for. Approval requirements for medical devices vary by. Medical devices, in japan, are classified into the following four categories based on their intended use and safety risk. How medical. Medical Device Classification In Japan.
From www.scas.co.jp
Development/Application Support Service for Medical Devices Medical Device Classification In Japan The mhlw uses a tiered classification system for. Medical devices, in japan, are classified into the following four categories based on their intended use and safety risk. Find out how to obtain. Learn about the regulatory authorities, classification, procedures and criteria for medical devices in japan. Approval requirements for medical devices vary by. Medical device classification and regulation. Medical devices. Medical Device Classification In Japan.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Medical Device Classification In Japan Medical devices, in japan, are classified into the following four categories based on their intended use and safety risk. Mds are classified into 4 categories (class i to iv) according to risk level. Learn about the regulatory authorities, classification, procedures and criteria for medical devices in japan. This web page provides information about the standards for medical devices in japan,. Medical Device Classification In Japan.
From mavink.com
Medical Device Classification Chart Medical Device Classification In Japan Learn about the regulatory authorities, classification, procedures and criteria for medical devices in japan. Medical devices, in japan, are classified into the following four categories based on their intended use and safety risk. Find out how to obtain. How medical devices are classified in japan. Medical device classification and regulation. The mhlw uses a tiered classification system for. Approval requirements. Medical Device Classification In Japan.
From credevo.com
Japan Medical Device Approval Process Credevo Articles Medical Device Classification In Japan Approval requirements for medical devices vary by. How medical devices are classified in japan. Learn about the regulatory authorities, classification, procedures and criteria for medical devices in japan. Medical devices in japan are classified based on risk to the human body. The mhlw uses a tiered classification system for. Jmdn is a system of classifying and identifying medical devices based. Medical Device Classification In Japan.
From www.freyrsolutions.com
Medical Device Riskclassification and Registration in Japan Freyr Medical Device Classification In Japan Medical devices in japan are classified based on risk to the human body. How medical devices are classified in japan. Mds are classified into 4 categories (class i to iv) according to risk level. Medical device classification and regulation. Approval requirements for medical devices vary by. Find out how to obtain. Jmdn is a system of classifying and identifying medical. Medical Device Classification In Japan.
From www.linkedin.com
Medical Device Classification In Japan Medical Device Classification In Japan Medical device classification and regulation. How medical devices are classified in japan. This web page provides information about the standards for medical devices in japan, such as certification criteria, nomenclature, and review. The mhlw uses a tiered classification system for. Learn about the regulatory authorities, classification, procedures and criteria for medical devices in japan. Medical devices in japan are classified. Medical Device Classification In Japan.
From www.slideshare.net
Japan medical device approval chart Emergo Group Medical Device Classification In Japan Mds are classified into 4 categories (class i to iv) according to risk level. The mhlw uses a tiered classification system for. Find out how to obtain. Medical devices in japan are classified based on risk to the human body. Medical device classification and regulation. This web page provides information about the standards for medical devices in japan, such as. Medical Device Classification In Japan.