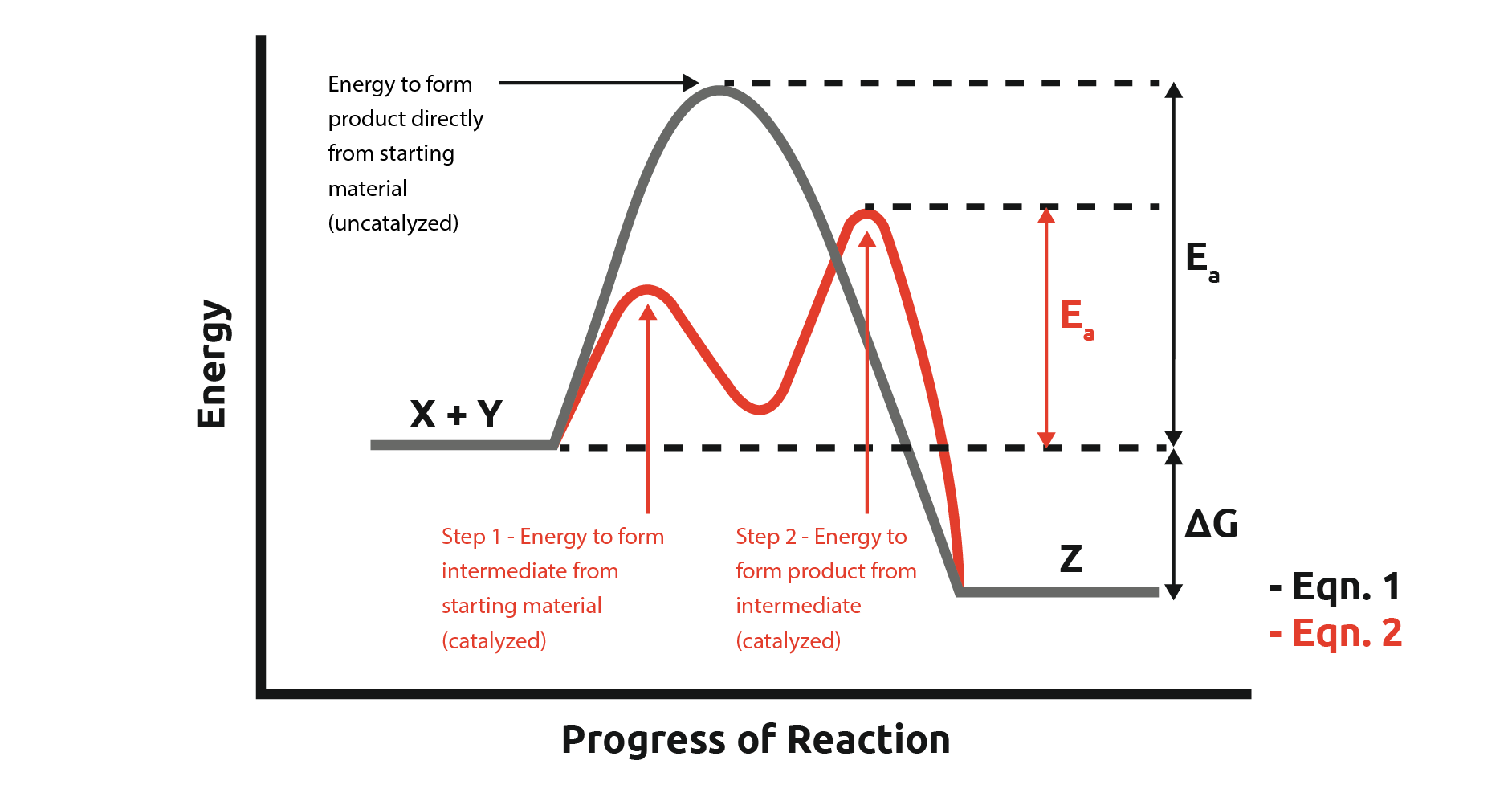
Catalysts Lower Activation Barrier . Catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be surmounted for a chemical. Excellent catalysts because they bind the transition states (ts) of the reaction they catalyze better than substrates and hence lower the. In heterogeneous catalysis, catalysts provide a surface to. Catalysts act by reducing the amount of activation energy needed for a successful collision between the reactant molecules. By lowering the activation energy barrier that often hinders reactions, catalysts usher reactions forward with renewed vigor,. There is a subtle difference. It does it by providing a partial bond which stabilises the transition. A catalyst can open a new reaction pathway with lower activation energy. A catalyst provides an alternative route for the reaction with a lower activation energy. In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. It does not lower the activation energy of the reaction.
from www.labunlimited.com
A catalyst can open a new reaction pathway with lower activation energy. In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to. Catalysts act by reducing the amount of activation energy needed for a successful collision between the reactant molecules. It does it by providing a partial bond which stabilises the transition. There is a subtle difference. Catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be surmounted for a chemical. It does not lower the activation energy of the reaction. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. By lowering the activation energy barrier that often hinders reactions, catalysts usher reactions forward with renewed vigor,. Excellent catalysts because they bind the transition states (ts) of the reaction they catalyze better than substrates and hence lower the.
Solid Phase Catalysis in Continuous Flow Chemistry Lab Unlimited
Catalysts Lower Activation Barrier It does not lower the activation energy of the reaction. It does not lower the activation energy of the reaction. Catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be surmounted for a chemical. Excellent catalysts because they bind the transition states (ts) of the reaction they catalyze better than substrates and hence lower the. In heterogeneous catalysis, catalysts provide a surface to. A catalyst can open a new reaction pathway with lower activation energy. A catalyst provides an alternative route for the reaction with a lower activation energy. It does it by providing a partial bond which stabilises the transition. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysts act by reducing the amount of activation energy needed for a successful collision between the reactant molecules. There is a subtle difference. In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to. By lowering the activation energy barrier that often hinders reactions, catalysts usher reactions forward with renewed vigor,.
From www.numerade.com
SOLVED Suppose that a catalyst lowers the activation barrier of a Catalysts Lower Activation Barrier It does it by providing a partial bond which stabilises the transition. Catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be surmounted for a chemical. It does not lower the activation energy of the reaction. A catalyst provides an alternative route for the reaction with a lower activation energy. In. Catalysts Lower Activation Barrier.
From www.chegg.com
Solved Suppose that a catalyst lowers the activation barrier Catalysts Lower Activation Barrier In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to. There is a subtle difference. By lowering the activation energy barrier that often hinders reactions, catalysts usher reactions forward with renewed vigor,. A catalyst provides an alternative route for the reaction with a lower activation energy. It. Catalysts Lower Activation Barrier.
From vdocuments.mx
Catalysts Catalystlowers activation energy of a chemical reaction and Catalysts Lower Activation Barrier By lowering the activation energy barrier that often hinders reactions, catalysts usher reactions forward with renewed vigor,. Excellent catalysts because they bind the transition states (ts) of the reaction they catalyze better than substrates and hence lower the. Catalysts act by reducing the amount of activation energy needed for a successful collision between the reactant molecules. Catalysts make this process. Catalysts Lower Activation Barrier.
From slideplayer.com
Enzymes. ppt download Catalysts Lower Activation Barrier Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to. In heterogeneous catalysis, catalysts provide a surface to. There is a subtle difference. Catalysts make this process more. Catalysts Lower Activation Barrier.
From www.slideserve.com
PPT Catalysts PowerPoint Presentation, free download ID2683737 Catalysts Lower Activation Barrier In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to. A catalyst can open a new reaction pathway with lower activation energy. Excellent catalysts because they bind the transition states (ts) of the reaction they catalyze better than substrates and hence lower the. It does it by. Catalysts Lower Activation Barrier.
From www.chegg.com
Solved A catalyst lowers the activation barrier by only Catalysts Lower Activation Barrier Excellent catalysts because they bind the transition states (ts) of the reaction they catalyze better than substrates and hence lower the. By lowering the activation energy barrier that often hinders reactions, catalysts usher reactions forward with renewed vigor,. Catalysts act by reducing the amount of activation energy needed for a successful collision between the reactant molecules. A catalyst provides an. Catalysts Lower Activation Barrier.
From www.slideserve.com
PPT Reaction Rates (Chapter 13) PowerPoint Presentation, free Catalysts Lower Activation Barrier In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to. Excellent catalysts because they bind the transition states (ts) of the reaction they catalyze better than substrates and hence lower the. Catalysts make this process more efficient by lowering the activation energy, which is the energy barrier. Catalysts Lower Activation Barrier.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalysts Lower Activation Barrier Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. It does not lower the activation energy of the reaction. In heterogeneous catalysis, catalysts provide a surface to. Excellent catalysts because they bind the transition states (ts) of the reaction they catalyze better than substrates and hence lower the. A catalyst. Catalysts Lower Activation Barrier.
From www.cheric.org
Chemical Reaction (Reaction rate) Catalysts Lower Activation Barrier It does it by providing a partial bond which stabilises the transition. Catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be surmounted for a chemical. A catalyst provides an alternative route for the reaction with a lower activation energy. Catalysts allow a reaction to proceed via a pathway that has. Catalysts Lower Activation Barrier.
From www.coursehero.com
[Solved] Suppose that a catalyst lowers the activation barrier of a Catalysts Lower Activation Barrier Catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be surmounted for a chemical. A catalyst can open a new reaction pathway with lower activation energy. There is a subtle difference. It does not lower the activation energy of the reaction. A catalyst provides an alternative route for the reaction with. Catalysts Lower Activation Barrier.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 3.2.4 Activation Energy翰林国际教育 Catalysts Lower Activation Barrier There is a subtle difference. Catalysts act by reducing the amount of activation energy needed for a successful collision between the reactant molecules. In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to. By lowering the activation energy barrier that often hinders reactions, catalysts usher reactions forward. Catalysts Lower Activation Barrier.
From slideplayer.com
Section 24 & 25 “Chemical Reactions & Enzymes” ppt download Catalysts Lower Activation Barrier Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be surmounted for a chemical. Catalysts act by reducing the amount of activation energy needed for a successful collision between the reactant molecules.. Catalysts Lower Activation Barrier.
From www.labunlimited.com
Solid Phase Catalysis in Continuous Flow Chemistry Lab Unlimited Catalysts Lower Activation Barrier In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to. Catalysts act by reducing the amount of activation energy needed for a successful collision between the reactant molecules. Excellent catalysts because they bind the transition states (ts) of the reaction they catalyze better than substrates and hence. Catalysts Lower Activation Barrier.
From www.coursehero.com
[Solved] Suppose that a catalyst lowers the activation barrier of a Catalysts Lower Activation Barrier In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to. There is a subtle difference. Catalysts act by reducing the amount of activation energy needed for a successful collision between the reactant molecules. A catalyst can open a new reaction pathway with lower activation energy. Excellent catalysts. Catalysts Lower Activation Barrier.
From www.sliderbase.com
Enzymes. A Cell's Catalysts Presentation Biology Catalysts Lower Activation Barrier There is a subtle difference. In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to. A catalyst provides an alternative route for the reaction with a lower activation energy. Catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must. Catalysts Lower Activation Barrier.
From gioyfzlvn.blob.core.windows.net
Which Catalysts Lower Activation Energy at Ronald Lerner blog Catalysts Lower Activation Barrier It does not lower the activation energy of the reaction. Catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be surmounted for a chemical. There is a subtle difference. In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary. Catalysts Lower Activation Barrier.
From www.numerade.com
Suppose that a catalyst lowers the activation barrier of a reaction Catalysts Lower Activation Barrier Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. A catalyst provides an alternative route for the reaction with a lower activation energy. By lowering the activation energy barrier that often hinders reactions, catalysts usher reactions forward with renewed vigor,. A catalyst can open a new reaction pathway with lower. Catalysts Lower Activation Barrier.
From www.chegg.com
Solved Suppose that a catalyst lowers the activation barrier Catalysts Lower Activation Barrier Catalysts act by reducing the amount of activation energy needed for a successful collision between the reactant molecules. A catalyst provides an alternative route for the reaction with a lower activation energy. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. In heterogeneous catalysis, catalysts provide a surface to. It. Catalysts Lower Activation Barrier.
From www.chim.lu
Activation energy and catalysis Catalysts Lower Activation Barrier It does not lower the activation energy of the reaction. There is a subtle difference. In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to. A catalyst can open a new reaction pathway with lower activation energy. Catalysts act by reducing the amount of activation energy needed. Catalysts Lower Activation Barrier.
From slideplayer.com
A catalyst lowers activation energy. ppt download Catalysts Lower Activation Barrier Catalysts act by reducing the amount of activation energy needed for a successful collision between the reactant molecules. A catalyst provides an alternative route for the reaction with a lower activation energy. It does it by providing a partial bond which stabilises the transition. Catalysts make this process more efficient by lowering the activation energy, which is the energy barrier. Catalysts Lower Activation Barrier.
From cheminfo.uz
Kataliz va katalizator haqida ChemInfo.uz Catalysts Lower Activation Barrier A catalyst can open a new reaction pathway with lower activation energy. It does not lower the activation energy of the reaction. In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to. Catalysts act by reducing the amount of activation energy needed for a successful collision between. Catalysts Lower Activation Barrier.
From www.numerade.com
Suppose that a catalyst lowers the activation barrier of a reaction Catalysts Lower Activation Barrier Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. It does not lower the activation energy of the reaction. In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to. Catalysts act by reducing the amount of activation. Catalysts Lower Activation Barrier.
From www.slideserve.com
PPT Reaction PowerPoint Presentation, free download ID1835084 Catalysts Lower Activation Barrier Excellent catalysts because they bind the transition states (ts) of the reaction they catalyze better than substrates and hence lower the. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. There is a subtle difference. Catalysts act by reducing the amount of activation energy needed for a successful collision between. Catalysts Lower Activation Barrier.
From slideplayer.com
A catalyst lowers activation energy. ppt download Catalysts Lower Activation Barrier It does not lower the activation energy of the reaction. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysts act by reducing the amount of activation energy needed for a successful collision between the reactant molecules. In the case of a biological reaction, when an enzyme (a form of. Catalysts Lower Activation Barrier.
From www.slideserve.com
PPT Mechanisms of Catalytic Reactions and Characterization of Catalysts Lower Activation Barrier Excellent catalysts because they bind the transition states (ts) of the reaction they catalyze better than substrates and hence lower the. A catalyst provides an alternative route for the reaction with a lower activation energy. In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to. In heterogeneous. Catalysts Lower Activation Barrier.
From news.mit.edu
Catalyst Symposium helps lower “activation barriers” for rising biology Catalysts Lower Activation Barrier By lowering the activation energy barrier that often hinders reactions, catalysts usher reactions forward with renewed vigor,. In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to. Catalysts act by reducing the amount of activation energy needed for a successful collision between the reactant molecules. A catalyst. Catalysts Lower Activation Barrier.
From interfaces.che.wisc.edu
Catalyzing Interest in Catalysis What is Catalysis? Gebbie Lab UW Catalysts Lower Activation Barrier Catalysts act by reducing the amount of activation energy needed for a successful collision between the reactant molecules. Catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be surmounted for a chemical. A catalyst provides an alternative route for the reaction with a lower activation energy. It does not lower the. Catalysts Lower Activation Barrier.
From www.chegg.com
Solved Suppose that a catalyst lowers the activation barrier Catalysts Lower Activation Barrier Catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be surmounted for a chemical. By lowering the activation energy barrier that often hinders reactions, catalysts usher reactions forward with renewed vigor,. In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation. Catalysts Lower Activation Barrier.
From slidetodoc.com
Enzymes are proteins that act as biological catalysts Catalysts Lower Activation Barrier There is a subtle difference. A catalyst can open a new reaction pathway with lower activation energy. A catalyst provides an alternative route for the reaction with a lower activation energy. By lowering the activation energy barrier that often hinders reactions, catalysts usher reactions forward with renewed vigor,. Excellent catalysts because they bind the transition states (ts) of the reaction. Catalysts Lower Activation Barrier.
From brainly.com
catalysts are molecules that lower the activation energy for a given Catalysts Lower Activation Barrier Catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be surmounted for a chemical. By lowering the activation energy barrier that often hinders reactions, catalysts usher reactions forward with renewed vigor,. Excellent catalysts because they bind the transition states (ts) of the reaction they catalyze better than substrates and hence lower. Catalysts Lower Activation Barrier.
From slideplayer.com
How Catalysts Work Main Concept ppt download Catalysts Lower Activation Barrier Excellent catalysts because they bind the transition states (ts) of the reaction they catalyze better than substrates and hence lower the. By lowering the activation energy barrier that often hinders reactions, catalysts usher reactions forward with renewed vigor,. Catalysts act by reducing the amount of activation energy needed for a successful collision between the reactant molecules. A catalyst provides an. Catalysts Lower Activation Barrier.
From brainly.com
Classify each statement about catalysts as true or false. Catalyst Catalysts Lower Activation Barrier In heterogeneous catalysis, catalysts provide a surface to. By lowering the activation energy barrier that often hinders reactions, catalysts usher reactions forward with renewed vigor,. There is a subtle difference. It does it by providing a partial bond which stabilises the transition. Excellent catalysts because they bind the transition states (ts) of the reaction they catalyze better than substrates and. Catalysts Lower Activation Barrier.
From www.chegg.com
Solved A catalyst lowers the activation barrier by only Catalysts Lower Activation Barrier In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to. By lowering the activation energy barrier that often hinders reactions, catalysts usher reactions forward with renewed vigor,. There is a subtle difference. A catalyst can open a new reaction pathway with lower activation energy. A catalyst provides. Catalysts Lower Activation Barrier.
From slideplayer.com
AP Bio Tuesday, Metabolic Reactions & Enzymes ppt download Catalysts Lower Activation Barrier It does not lower the activation energy of the reaction. Catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be surmounted for a chemical. By lowering the activation energy barrier that often hinders reactions, catalysts usher reactions forward with renewed vigor,. It does it by providing a partial bond which stabilises. Catalysts Lower Activation Barrier.
From www.chegg.com
Solved A catalyst lowers the activation barrier by only Catalysts Lower Activation Barrier A catalyst provides an alternative route for the reaction with a lower activation energy. In the case of a biological reaction, when an enzyme (a form of catalyst) binds to a substrate, the activation energy necessary to. It does it by providing a partial bond which stabilises the transition. In heterogeneous catalysis, catalysts provide a surface to. There is a. Catalysts Lower Activation Barrier.