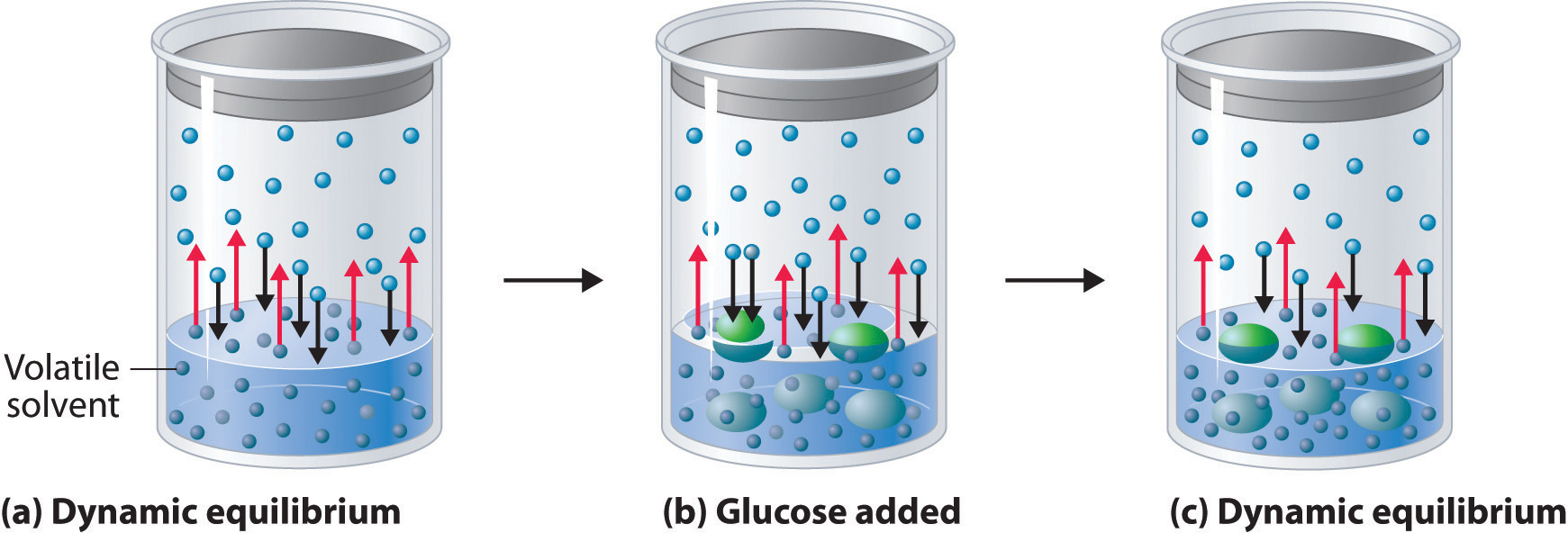
Vapour Pressure Of Solid In Liquid Solution . That is, the pressure of the vapor resulting from. another important property of liquids (and solids) that is governed by intermolecular forces is vapor pressure. a liquid’s vapour pressure is a vapour’s equilibrium pressure above its liquid (or solid); we can solve vapor pressure problems in either of two ways: By using equation \ref{13.6.1} to calculate the actual vapor. Vapour pressure can be defined as pressure formed by the vapor of the liquid (or solid) over the surface of the liquid. Vapor pressure is defined as the partial pressure of a. the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); vapour pressure of liquid solutions. at any given temperature for a particular solid or liquid, there is a pressure at which the vapor formed above the.
from chem.libretexts.org
Vapor pressure is defined as the partial pressure of a. we can solve vapor pressure problems in either of two ways: the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); at any given temperature for a particular solid or liquid, there is a pressure at which the vapor formed above the. a liquid’s vapour pressure is a vapour’s equilibrium pressure above its liquid (or solid); another important property of liquids (and solids) that is governed by intermolecular forces is vapor pressure. vapour pressure of liquid solutions. Vapour pressure can be defined as pressure formed by the vapor of the liquid (or solid) over the surface of the liquid. By using equation \ref{13.6.1} to calculate the actual vapor. That is, the pressure of the vapor resulting from.
11.5 Phase Equilibrium in Solutions Nonvolatile Solutes Chemistry LibreTexts
Vapour Pressure Of Solid In Liquid Solution at any given temperature for a particular solid or liquid, there is a pressure at which the vapor formed above the. another important property of liquids (and solids) that is governed by intermolecular forces is vapor pressure. at any given temperature for a particular solid or liquid, there is a pressure at which the vapor formed above the. the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); That is, the pressure of the vapor resulting from. we can solve vapor pressure problems in either of two ways: vapour pressure of liquid solutions. Vapor pressure is defined as the partial pressure of a. By using equation \ref{13.6.1} to calculate the actual vapor. a liquid’s vapour pressure is a vapour’s equilibrium pressure above its liquid (or solid); Vapour pressure can be defined as pressure formed by the vapor of the liquid (or solid) over the surface of the liquid.
From chem.libretexts.org
11.5 Vapor Pressure Chemistry LibreTexts Vapour Pressure Of Solid In Liquid Solution Vapor pressure is defined as the partial pressure of a. vapour pressure of liquid solutions. Vapour pressure can be defined as pressure formed by the vapor of the liquid (or solid) over the surface of the liquid. the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); By using equation. Vapour Pressure Of Solid In Liquid Solution.
From www.youtube.com
Raoult's law,Vapour pressure of solid in liquid solutions, explained in malayalam YouTube Vapour Pressure Of Solid In Liquid Solution vapour pressure of liquid solutions. That is, the pressure of the vapor resulting from. we can solve vapor pressure problems in either of two ways: a liquid’s vapour pressure is a vapour’s equilibrium pressure above its liquid (or solid); Vapor pressure is defined as the partial pressure of a. at any given temperature for a particular. Vapour Pressure Of Solid In Liquid Solution.
From www.youtube.com
Lecture06 Vapor pressure of solid in Liquid solutions. YouTube Vapour Pressure Of Solid In Liquid Solution at any given temperature for a particular solid or liquid, there is a pressure at which the vapor formed above the. another important property of liquids (and solids) that is governed by intermolecular forces is vapor pressure. the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Vapor pressure. Vapour Pressure Of Solid In Liquid Solution.
From derangedphysiology.com
Physics of humidity and evaporation Deranged Physiology Vapour Pressure Of Solid In Liquid Solution Vapour pressure can be defined as pressure formed by the vapor of the liquid (or solid) over the surface of the liquid. That is, the pressure of the vapor resulting from. a liquid’s vapour pressure is a vapour’s equilibrium pressure above its liquid (or solid); we can solve vapor pressure problems in either of two ways: the. Vapour Pressure Of Solid In Liquid Solution.
From learninggrabstein7j.z14.web.core.windows.net
Vapor Pressure And Boiling Worksheets Vapour Pressure Of Solid In Liquid Solution we can solve vapor pressure problems in either of two ways: Vapor pressure is defined as the partial pressure of a. the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); a liquid’s vapour pressure is a vapour’s equilibrium pressure above its liquid (or solid); vapour pressure of. Vapour Pressure Of Solid In Liquid Solution.
From www.youtube.com
Vapour Pressure Of Liquid In Liquid And Solids In Liquid Solutions/Raoult's Law /Class 12 Vapour Pressure Of Solid In Liquid Solution the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); By using equation \ref{13.6.1} to calculate the actual vapor. a liquid’s vapour pressure is a vapour’s equilibrium pressure above its liquid (or solid); at any given temperature for a particular solid or liquid, there is a pressure at which. Vapour Pressure Of Solid In Liquid Solution.
From nigerianscholars.com
Vapor Pressure Lowering Solutions and Colloids Vapour Pressure Of Solid In Liquid Solution at any given temperature for a particular solid or liquid, there is a pressure at which the vapor formed above the. Vapor pressure is defined as the partial pressure of a. a liquid’s vapour pressure is a vapour’s equilibrium pressure above its liquid (or solid); Vapour pressure can be defined as pressure formed by the vapor of the. Vapour Pressure Of Solid In Liquid Solution.
From byjus.com
the vapour pressure of pure liquids Aand B are 200 and 350 mm hg respectively , at 300k . mole Vapour Pressure Of Solid In Liquid Solution That is, the pressure of the vapor resulting from. Vapour pressure can be defined as pressure formed by the vapor of the liquid (or solid) over the surface of the liquid. at any given temperature for a particular solid or liquid, there is a pressure at which the vapor formed above the. a liquid’s vapour pressure is a. Vapour Pressure Of Solid In Liquid Solution.
From chem.libretexts.org
11.5 Phase Equilibrium in Solutions Nonvolatile Solutes Chemistry LibreTexts Vapour Pressure Of Solid In Liquid Solution That is, the pressure of the vapor resulting from. By using equation \ref{13.6.1} to calculate the actual vapor. we can solve vapor pressure problems in either of two ways: Vapor pressure is defined as the partial pressure of a. another important property of liquids (and solids) that is governed by intermolecular forces is vapor pressure. the vapor. Vapour Pressure Of Solid In Liquid Solution.
From www.youtube.com
Vapour Pressure of Liquid Solutions Solution and Colligative Properties Chemistry Class 12 Vapour Pressure Of Solid In Liquid Solution we can solve vapor pressure problems in either of two ways: Vapor pressure is defined as the partial pressure of a. the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); another important property of liquids (and solids) that is governed by intermolecular forces is vapor pressure. That is,. Vapour Pressure Of Solid In Liquid Solution.
From www.brainkart.com
Vapour pressure of liquid solutions Chemistry Vapour Pressure Of Solid In Liquid Solution By using equation \ref{13.6.1} to calculate the actual vapor. Vapour pressure can be defined as pressure formed by the vapor of the liquid (or solid) over the surface of the liquid. we can solve vapor pressure problems in either of two ways: at any given temperature for a particular solid or liquid, there is a pressure at which. Vapour Pressure Of Solid In Liquid Solution.
From sciencenotes.org
Vapor Pressure Definition and How to Calculate It Vapour Pressure Of Solid In Liquid Solution That is, the pressure of the vapor resulting from. we can solve vapor pressure problems in either of two ways: another important property of liquids (and solids) that is governed by intermolecular forces is vapor pressure. By using equation \ref{13.6.1} to calculate the actual vapor. Vapor pressure is defined as the partial pressure of a. Vapour pressure can. Vapour Pressure Of Solid In Liquid Solution.
From www.youtube.com
vapour pressure of liquids solution solutions class 12 chemistry subject cbse YouTube Vapour Pressure Of Solid In Liquid Solution That is, the pressure of the vapor resulting from. another important property of liquids (and solids) that is governed by intermolecular forces is vapor pressure. By using equation \ref{13.6.1} to calculate the actual vapor. we can solve vapor pressure problems in either of two ways: Vapor pressure is defined as the partial pressure of a. vapour pressure. Vapour Pressure Of Solid In Liquid Solution.
From www.youtube.com
Vapour Pressure of Solid in Liquid Solutions Numericals Umang Class 12 CBSE+JEE+NEET 2022 Vapour Pressure Of Solid In Liquid Solution another important property of liquids (and solids) that is governed by intermolecular forces is vapor pressure. vapour pressure of liquid solutions. a liquid’s vapour pressure is a vapour’s equilibrium pressure above its liquid (or solid); That is, the pressure of the vapor resulting from. we can solve vapor pressure problems in either of two ways: . Vapour Pressure Of Solid In Liquid Solution.
From learningmagichazel.z13.web.core.windows.net
Solid Liquid And Gas Lesson Vapour Pressure Of Solid In Liquid Solution Vapor pressure is defined as the partial pressure of a. at any given temperature for a particular solid or liquid, there is a pressure at which the vapor formed above the. Vapour pressure can be defined as pressure formed by the vapor of the liquid (or solid) over the surface of the liquid. By using equation \ref{13.6.1} to calculate. Vapour Pressure Of Solid In Liquid Solution.
From www.youtube.com
Vapour Pressure of Solid Liquid Solution Class 12th Chapter 2 Solution Part 17 YouTube Vapour Pressure Of Solid In Liquid Solution the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); we can solve vapor pressure problems in either of two ways: Vapor pressure is defined as the partial pressure of a. at any given temperature for a particular solid or liquid, there is a pressure at which the vapor. Vapour Pressure Of Solid In Liquid Solution.
From www.chegg.com
Solved The Vapor Pressure Of Solid NaF Varies With Temper... Vapour Pressure Of Solid In Liquid Solution By using equation \ref{13.6.1} to calculate the actual vapor. Vapour pressure can be defined as pressure formed by the vapor of the liquid (or solid) over the surface of the liquid. at any given temperature for a particular solid or liquid, there is a pressure at which the vapor formed above the. a liquid’s vapour pressure is a. Vapour Pressure Of Solid In Liquid Solution.
From surfacemeasurementsystems.com
Vapor Pressure of Solids and Liquids Sorption Applications Vapour Pressure Of Solid In Liquid Solution we can solve vapor pressure problems in either of two ways: the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); By using equation \ref{13.6.1} to calculate the actual vapor. at any given temperature for a particular solid or liquid, there is a pressure at which the vapor formed. Vapour Pressure Of Solid In Liquid Solution.
From www.slideserve.com
PPT Chapter 17 PowerPoint Presentation ID6750477 Vapour Pressure Of Solid In Liquid Solution another important property of liquids (and solids) that is governed by intermolecular forces is vapor pressure. vapour pressure of liquid solutions. That is, the pressure of the vapor resulting from. a liquid’s vapour pressure is a vapour’s equilibrium pressure above its liquid (or solid); Vapour pressure can be defined as pressure formed by the vapor of the. Vapour Pressure Of Solid In Liquid Solution.
From www.youtube.com
Solutions L04 Vapour Pressure of SolidLiquid Solutions CL126 Free Chemistry Lectures for Neet Vapour Pressure Of Solid In Liquid Solution another important property of liquids (and solids) that is governed by intermolecular forces is vapor pressure. Vapour pressure can be defined as pressure formed by the vapor of the liquid (or solid) over the surface of the liquid. we can solve vapor pressure problems in either of two ways: By using equation \ref{13.6.1} to calculate the actual vapor.. Vapour Pressure Of Solid In Liquid Solution.
From www.slideserve.com
PPT I. The Vapor Pressure of Solutions PowerPoint Presentation, free download ID1815834 Vapour Pressure Of Solid In Liquid Solution By using equation \ref{13.6.1} to calculate the actual vapor. a liquid’s vapour pressure is a vapour’s equilibrium pressure above its liquid (or solid); the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Vapor pressure is defined as the partial pressure of a. vapour pressure of liquid solutions. . Vapour Pressure Of Solid In Liquid Solution.
From thechemistrynotes.com
Vapor Pressure of LiquidLiquid Solution Vapour Pressure Of Solid In Liquid Solution at any given temperature for a particular solid or liquid, there is a pressure at which the vapor formed above the. we can solve vapor pressure problems in either of two ways: That is, the pressure of the vapor resulting from. vapour pressure of liquid solutions. Vapor pressure is defined as the partial pressure of a. . Vapour Pressure Of Solid In Liquid Solution.
From www.slideserve.com
PPT Vapor Pressure of Solutions PowerPoint Presentation, free download ID6781569 Vapour Pressure Of Solid In Liquid Solution Vapor pressure is defined as the partial pressure of a. another important property of liquids (and solids) that is governed by intermolecular forces is vapor pressure. the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); we can solve vapor pressure problems in either of two ways: That is,. Vapour Pressure Of Solid In Liquid Solution.
From www.youtube.com
Substitutional & Interstitial Solid Solution, Vapour pressure, lowering of vapour pressure by A Vapour Pressure Of Solid In Liquid Solution another important property of liquids (and solids) that is governed by intermolecular forces is vapor pressure. the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); at any given temperature for a particular solid or liquid, there is a pressure at which the vapor formed above the. vapour. Vapour Pressure Of Solid In Liquid Solution.
From www.doubtnut.com
[Gujrati] Explain vapour pressure of solutions of solids in liquids. Vapour Pressure Of Solid In Liquid Solution we can solve vapor pressure problems in either of two ways: another important property of liquids (and solids) that is governed by intermolecular forces is vapor pressure. at any given temperature for a particular solid or liquid, there is a pressure at which the vapor formed above the. Vapour pressure can be defined as pressure formed by. Vapour Pressure Of Solid In Liquid Solution.
From www.youtube.com
Vapour Pressure Of Solids In Liquid Solution In Tamil Class 12 Solutions YouTube Vapour Pressure Of Solid In Liquid Solution Vapour pressure can be defined as pressure formed by the vapor of the liquid (or solid) over the surface of the liquid. By using equation \ref{13.6.1} to calculate the actual vapor. we can solve vapor pressure problems in either of two ways: vapour pressure of liquid solutions. Vapor pressure is defined as the partial pressure of a. . Vapour Pressure Of Solid In Liquid Solution.
From www.slideserve.com
PPT Solution properties PowerPoint Presentation, free download ID5913365 Vapour Pressure Of Solid In Liquid Solution at any given temperature for a particular solid or liquid, there is a pressure at which the vapor formed above the. That is, the pressure of the vapor resulting from. a liquid’s vapour pressure is a vapour’s equilibrium pressure above its liquid (or solid); Vapour pressure can be defined as pressure formed by the vapor of the liquid. Vapour Pressure Of Solid In Liquid Solution.
From www.youtube.com
VAPOUR PRESSURE OF LIQUID SOLUTIONS YouTube Vapour Pressure Of Solid In Liquid Solution Vapor pressure is defined as the partial pressure of a. another important property of liquids (and solids) that is governed by intermolecular forces is vapor pressure. at any given temperature for a particular solid or liquid, there is a pressure at which the vapor formed above the. Vapour pressure can be defined as pressure formed by the vapor. Vapour Pressure Of Solid In Liquid Solution.
From www.chegg.com
Solved The vapor pressure of SO2 in solid and liquid form, Vapour Pressure Of Solid In Liquid Solution vapour pressure of liquid solutions. By using equation \ref{13.6.1} to calculate the actual vapor. we can solve vapor pressure problems in either of two ways: a liquid’s vapour pressure is a vapour’s equilibrium pressure above its liquid (or solid); another important property of liquids (and solids) that is governed by intermolecular forces is vapor pressure. Vapor. Vapour Pressure Of Solid In Liquid Solution.
From www.youtube.com
5 5 Solids Liquids Vapor Pressure YouTube Vapour Pressure Of Solid In Liquid Solution Vapour pressure can be defined as pressure formed by the vapor of the liquid (or solid) over the surface of the liquid. Vapor pressure is defined as the partial pressure of a. the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); at any given temperature for a particular solid. Vapour Pressure Of Solid In Liquid Solution.
From www.youtube.com
vapour pressure of liquids solutions solutions class 12 chemistry subject notes lectures cbse Vapour Pressure Of Solid In Liquid Solution another important property of liquids (and solids) that is governed by intermolecular forces is vapor pressure. a liquid’s vapour pressure is a vapour’s equilibrium pressure above its liquid (or solid); vapour pressure of liquid solutions. Vapour pressure can be defined as pressure formed by the vapor of the liquid (or solid) over the surface of the liquid.. Vapour Pressure Of Solid In Liquid Solution.
From www.brainkart.com
Vapour pressure of liquid Solutions Chemistry Vapour Pressure Of Solid In Liquid Solution Vapour pressure can be defined as pressure formed by the vapor of the liquid (or solid) over the surface of the liquid. That is, the pressure of the vapor resulting from. another important property of liquids (and solids) that is governed by intermolecular forces is vapor pressure. By using equation \ref{13.6.1} to calculate the actual vapor. a liquid’s. Vapour Pressure Of Solid In Liquid Solution.
From scienceinfo.com
Vapor Pressure of LiquidLiquid Solution Vapour Pressure Of Solid In Liquid Solution That is, the pressure of the vapor resulting from. another important property of liquids (and solids) that is governed by intermolecular forces is vapor pressure. vapour pressure of liquid solutions. Vapour pressure can be defined as pressure formed by the vapor of the liquid (or solid) over the surface of the liquid. the vapor pressure of a. Vapour Pressure Of Solid In Liquid Solution.
From kunduz.com
[ANSWERED] Physics The vapour pressure of a solution having solid as Kunduz Vapour Pressure Of Solid In Liquid Solution we can solve vapor pressure problems in either of two ways: the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); another important property of liquids (and solids) that is governed by intermolecular forces is vapor pressure. Vapour pressure can be defined as pressure formed by the vapor of. Vapour Pressure Of Solid In Liquid Solution.
From www.researchgate.net
Vapour pressure curve (solid line), critical point (black circle) and... Download Scientific Vapour Pressure Of Solid In Liquid Solution at any given temperature for a particular solid or liquid, there is a pressure at which the vapor formed above the. the vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); By using equation \ref{13.6.1} to calculate the actual vapor. That is, the pressure of the vapor resulting from. Vapour. Vapour Pressure Of Solid In Liquid Solution.