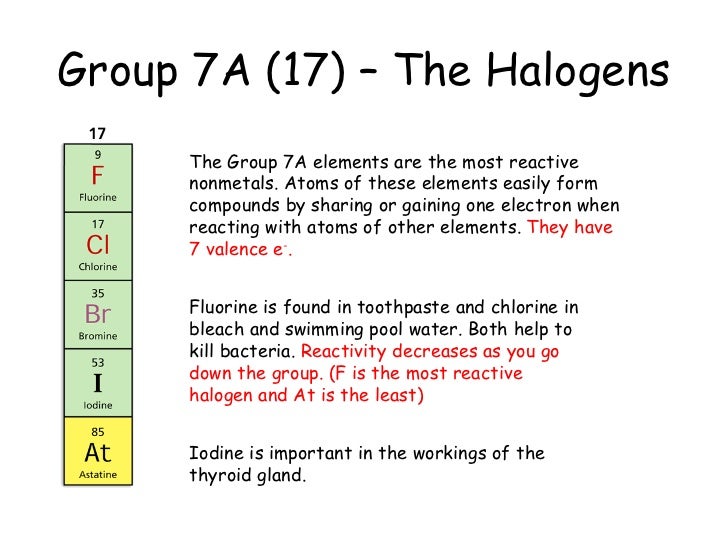
What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine . Reacts with heated iron wool very quickly, although not as quickly as fluorine does. The order of reactivity is chlorine > bromine > iodine. The great reactivity of fluorine largely stems from the relatively low dissociation. It is the weakest oxidizing agent, and the iodide ion is the most easily oxidized. use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. the halogens are elements belonging to group 7a.fluorine, chlorine, bromine, and iodine can be added to. as a general rule, fluorine is the most reactive halogen and astatine is the least reactive. you can use these results to place the three halogens in order of reactivity: All halogens form group 1. A more reactive halogen can displace a less reactive one from a. Chlorine is the most reactive because it can displace. iodine is the least reactive of the halogens. halogens can be dissolved in water to form acidic solutions.
from www.slideshare.net
A more reactive halogen can displace a less reactive one from a. The great reactivity of fluorine largely stems from the relatively low dissociation. iodine is the least reactive of the halogens. as a general rule, fluorine is the most reactive halogen and astatine is the least reactive. It is the weakest oxidizing agent, and the iodide ion is the most easily oxidized. All halogens form group 1. the halogens are elements belonging to group 7a.fluorine, chlorine, bromine, and iodine can be added to. use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. The order of reactivity is chlorine > bromine > iodine. you can use these results to place the three halogens in order of reactivity:
The periodic table
What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine Reacts with heated iron wool very quickly, although not as quickly as fluorine does. the halogens are elements belonging to group 7a.fluorine, chlorine, bromine, and iodine can be added to. The great reactivity of fluorine largely stems from the relatively low dissociation. The order of reactivity is chlorine > bromine > iodine. halogens can be dissolved in water to form acidic solutions. Chlorine is the most reactive because it can displace. as a general rule, fluorine is the most reactive halogen and astatine is the least reactive. iodine is the least reactive of the halogens. A more reactive halogen can displace a less reactive one from a. you can use these results to place the three halogens in order of reactivity: All halogens form group 1. It is the weakest oxidizing agent, and the iodide ion is the most easily oxidized. use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. Reacts with heated iron wool very quickly, although not as quickly as fluorine does.
From in.pinterest.com
Comparing halogen reactivity trends with those of the alkali metals. Halogens less What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine A more reactive halogen can displace a less reactive one from a. Chlorine is the most reactive because it can displace. use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. The order of reactivity is chlorine > bromine > iodine. Reacts with heated iron wool very quickly, although not as. What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine.
From www.youtube.com
Group 7 Elements. Halogens. Salt Formers. Fluorine, Chlorine, Bromine, Iodine, Astatine. YouTube What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine Reacts with heated iron wool very quickly, although not as quickly as fluorine does. A more reactive halogen can displace a less reactive one from a. as a general rule, fluorine is the most reactive halogen and astatine is the least reactive. the halogens are elements belonging to group 7a.fluorine, chlorine, bromine, and iodine can be added to.. What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine.
From material-properties.org
Chlorine and Bromine Comparison Properties Material Properties What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine All halogens form group 1. A more reactive halogen can displace a less reactive one from a. iodine is the least reactive of the halogens. as a general rule, fluorine is the most reactive halogen and astatine is the least reactive. The order of reactivity is chlorine > bromine > iodine. The great reactivity of fluorine largely stems. What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine.
From www.sliderbase.com
Group 7, The Halogens Presentation Chemistry What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine The great reactivity of fluorine largely stems from the relatively low dissociation. use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. It is the weakest oxidizing agent, and the iodide ion is the most easily oxidized. A more reactive halogen can displace a less reactive one from a. Chlorine is. What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine.
From ecurrencythailand.com
Which Is The Least Reactive Fluorine Chlorine Bromine Iodine? 22 Most Correct Answers What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine iodine is the least reactive of the halogens. the halogens are elements belonging to group 7a.fluorine, chlorine, bromine, and iodine can be added to. halogens can be dissolved in water to form acidic solutions. All halogens form group 1. A more reactive halogen can displace a less reactive one from a. Reacts with heated iron wool very. What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine.
From chemizi.blogspot.com
Halogen elementsdefinitionpropertiesreactivity and uses. What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine as a general rule, fluorine is the most reactive halogen and astatine is the least reactive. The great reactivity of fluorine largely stems from the relatively low dissociation. The order of reactivity is chlorine > bromine > iodine. use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. you. What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine.
From slideplayer.com
Chemical Families. ppt download What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine The great reactivity of fluorine largely stems from the relatively low dissociation. All halogens form group 1. Chlorine is the most reactive because it can displace. The order of reactivity is chlorine > bromine > iodine. Reacts with heated iron wool very quickly, although not as quickly as fluorine does. you can use these results to place the three. What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine.
From pdfslide.net
(PPT) Starter 1) Which is the most reactive halogen? bromine chlorine fluorine iodine 2) How What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. Reacts with heated iron wool very quickly, although not as quickly as fluorine does. Chlorine is the most reactive because it can displace. It is the weakest oxidizing agent, and the iodide ion is the most easily oxidized. you can. What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Halogen What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. Chlorine is the most reactive because it can displace. It is the weakest oxidizing agent, and the iodide ion is the most easily oxidized. iodine is the least reactive of the halogens. All halogens form group 1. The order of. What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine.
From slideplayer.com
Chapter 8 Chemical Equations. ppt download What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine The great reactivity of fluorine largely stems from the relatively low dissociation. the halogens are elements belonging to group 7a.fluorine, chlorine, bromine, and iodine can be added to. you can use these results to place the three halogens in order of reactivity: The order of reactivity is chlorine > bromine > iodine. It is the weakest oxidizing agent,. What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine.
From byjus.com
Electronic Configuration Halogen Characterisitcs Periodic Table What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine halogens can be dissolved in water to form acidic solutions. as a general rule, fluorine is the most reactive halogen and astatine is the least reactive. Reacts with heated iron wool very quickly, although not as quickly as fluorine does. The great reactivity of fluorine largely stems from the relatively low dissociation. The order of reactivity is chlorine. What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine.
From www.thoughtco.com
List of Halogens (Element Groups) What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine A more reactive halogen can displace a less reactive one from a. All halogens form group 1. Chlorine is the most reactive because it can displace. The order of reactivity is chlorine > bromine > iodine. Reacts with heated iron wool very quickly, although not as quickly as fluorine does. as a general rule, fluorine is the most reactive. What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine.
From materikimia.com
Nama Lain dari Golongan 1A Sampai 8A Serta Sifat Unsurnya Materi Kimia What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine The great reactivity of fluorine largely stems from the relatively low dissociation. use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. as a general rule, fluorine is the most reactive halogen and astatine is the least reactive. Reacts with heated iron wool very quickly, although not as quickly as. What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine.
From laserrolf.weebly.com
laserrolf Blog What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine as a general rule, fluorine is the most reactive halogen and astatine is the least reactive. Reacts with heated iron wool very quickly, although not as quickly as fluorine does. All halogens form group 1. It is the weakest oxidizing agent, and the iodide ion is the most easily oxidized. The order of reactivity is chlorine > bromine >. What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine.
From www.youtube.com
THE HALOGENS FLUORINE, CHLORINE, BROMINE, IODINE AND ASTATINE. YouTube What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine as a general rule, fluorine is the most reactive halogen and astatine is the least reactive. The order of reactivity is chlorine > bromine > iodine. you can use these results to place the three halogens in order of reactivity: the halogens are elements belonging to group 7a.fluorine, chlorine, bromine, and iodine can be added to. . What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine.
From www.slideserve.com
PPT Halogens PowerPoint Presentation, free download ID6732639 What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine Reacts with heated iron wool very quickly, although not as quickly as fluorine does. It is the weakest oxidizing agent, and the iodide ion is the most easily oxidized. the halogens are elements belonging to group 7a.fluorine, chlorine, bromine, and iodine can be added to. Chlorine is the most reactive because it can displace. iodine is the least. What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine.
From www.slideserve.com
PPT Chemistry UNIT 3 PowerPoint Presentation, free download ID5906142 What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine A more reactive halogen can displace a less reactive one from a. Chlorine is the most reactive because it can displace. use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. iodine is the least reactive of the halogens. All halogens form group 1. The order of reactivity is chlorine. What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine.
From slideplayer.com
Which of the elements shown has 1 outer electron (D1)? ppt download What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine iodine is the least reactive of the halogens. you can use these results to place the three halogens in order of reactivity: halogens can be dissolved in water to form acidic solutions. the halogens are elements belonging to group 7a.fluorine, chlorine, bromine, and iodine can be added to. as a general rule, fluorine is the. What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine.
From pediaa.com
Difference Between Bromine and Chlorine What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine The order of reactivity is chlorine > bromine > iodine. Reacts with heated iron wool very quickly, although not as quickly as fluorine does. iodine is the least reactive of the halogens. use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. All halogens form group 1. A more reactive. What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine.
From www.sliderbase.com
Group 7, The Halogens Presentation Chemistry What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine The order of reactivity is chlorine > bromine > iodine. The great reactivity of fluorine largely stems from the relatively low dissociation. iodine is the least reactive of the halogens. Chlorine is the most reactive because it can displace. A more reactive halogen can displace a less reactive one from a. the halogens are elements belonging to group. What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine.
From www.slideserve.com
PPT Group 7 the halogens PowerPoint Presentation, free download ID3005113 What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine iodine is the least reactive of the halogens. Chlorine is the most reactive because it can displace. It is the weakest oxidizing agent, and the iodide ion is the most easily oxidized. A more reactive halogen can displace a less reactive one from a. the halogens are elements belonging to group 7a.fluorine, chlorine, bromine, and iodine can be. What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine.
From www.learnatnoon.com
Which Halogen Has The Lowest Electronegativity? Noon Academy What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine It is the weakest oxidizing agent, and the iodide ion is the most easily oxidized. The order of reactivity is chlorine > bromine > iodine. you can use these results to place the three halogens in order of reactivity: The great reactivity of fluorine largely stems from the relatively low dissociation. as a general rule, fluorine is the. What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine.
From www.vedantu.com
Halogens Learn Definition, Properties, Facts & Examples What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine Reacts with heated iron wool very quickly, although not as quickly as fluorine does. you can use these results to place the three halogens in order of reactivity: It is the weakest oxidizing agent, and the iodide ion is the most easily oxidized. iodine is the least reactive of the halogens. A more reactive halogen can displace a. What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine.
From slideplayer.com
Elements, Atoms & Ions Chapter 4 ppt download What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine The great reactivity of fluorine largely stems from the relatively low dissociation. A more reactive halogen can displace a less reactive one from a. Chlorine is the most reactive because it can displace. as a general rule, fluorine is the most reactive halogen and astatine is the least reactive. It is the weakest oxidizing agent, and the iodide ion. What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine.
From www.slideserve.com
PPT Halogens PowerPoint Presentation, free download ID2110165 What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine Reacts with heated iron wool very quickly, although not as quickly as fluorine does. halogens can be dissolved in water to form acidic solutions. It is the weakest oxidizing agent, and the iodide ion is the most easily oxidized. the halogens are elements belonging to group 7a.fluorine, chlorine, bromine, and iodine can be added to. iodine is. What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine.
From sciencestruck.com
Bromine Vs. Chlorine What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine halogens can be dissolved in water to form acidic solutions. It is the weakest oxidizing agent, and the iodide ion is the most easily oxidized. Reacts with heated iron wool very quickly, although not as quickly as fluorine does. A more reactive halogen can displace a less reactive one from a. the halogens are elements belonging to group. What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine.
From sciencenotes.org
Halogen Elements List and Facts What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine Chlorine is the most reactive because it can displace. The great reactivity of fluorine largely stems from the relatively low dissociation. the halogens are elements belonging to group 7a.fluorine, chlorine, bromine, and iodine can be added to. The order of reactivity is chlorine > bromine > iodine. use the results in the table to deduce an order of. What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine.
From www.thoughtco.com
Halogen Elements and Properties What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine iodine is the least reactive of the halogens. use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. The great reactivity of fluorine largely stems from the relatively low dissociation. Reacts with heated iron wool very quickly, although not as quickly as fluorine does. The order of reactivity is chlorine. What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine.
From slidetodoc.com
HALOGENS Chlorine Fluorine GROUP 7 A Bromine Iodine What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine The great reactivity of fluorine largely stems from the relatively low dissociation. The order of reactivity is chlorine > bromine > iodine. as a general rule, fluorine is the most reactive halogen and astatine is the least reactive. iodine is the least reactive of the halogens. Chlorine is the most reactive because it can displace. use the. What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine.
From www.slideshare.net
The periodic table What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine Chlorine is the most reactive because it can displace. you can use these results to place the three halogens in order of reactivity: The great reactivity of fluorine largely stems from the relatively low dissociation. use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. A more reactive halogen can. What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine.
From www.slideserve.com
PPT Reactions of the halogens and halide ions PowerPoint Presentation ID2518013 What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine halogens can be dissolved in water to form acidic solutions. the halogens are elements belonging to group 7a.fluorine, chlorine, bromine, and iodine can be added to. iodine is the least reactive of the halogens. as a general rule, fluorine is the most reactive halogen and astatine is the least reactive. The great reactivity of fluorine largely. What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine.
From barkmanoil.com
Why Is Chlorine More Reactive Than Bromine? The 8 Top Answers What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. The great reactivity of fluorine largely stems from the relatively low dissociation. Chlorine is the most reactive because it can displace. halogens can be dissolved in water to form acidic solutions. It is the weakest oxidizing agent, and the iodide. What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine.
From www.sliderbase.com
The Halogens Presentation Chemistry What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine Chlorine is the most reactive because it can displace. Reacts with heated iron wool very quickly, although not as quickly as fluorine does. iodine is the least reactive of the halogens. A more reactive halogen can displace a less reactive one from a. halogens can be dissolved in water to form acidic solutions. you can use these. What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine.
From www.slideserve.com
PPT Section 52 Electron Configuration and the Periodic Table PowerPoint Presentation ID What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine It is the weakest oxidizing agent, and the iodide ion is the most easily oxidized. the halogens are elements belonging to group 7a.fluorine, chlorine, bromine, and iodine can be added to. The order of reactivity is chlorine > bromine > iodine. A more reactive halogen can displace a less reactive one from a. All halogens form group 1. . What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine.
From chemisfast.blogspot.com
Halogen family elementspropertiesperiodic tableoxyacidsradioactivity. PG.CHEMEASY What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine as a general rule, fluorine is the most reactive halogen and astatine is the least reactive. Reacts with heated iron wool very quickly, although not as quickly as fluorine does. use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. A more reactive halogen can displace a less reactive one. What Is The Least Reactive Halogen Out Of Chlorine Fluorine Or Bromine.