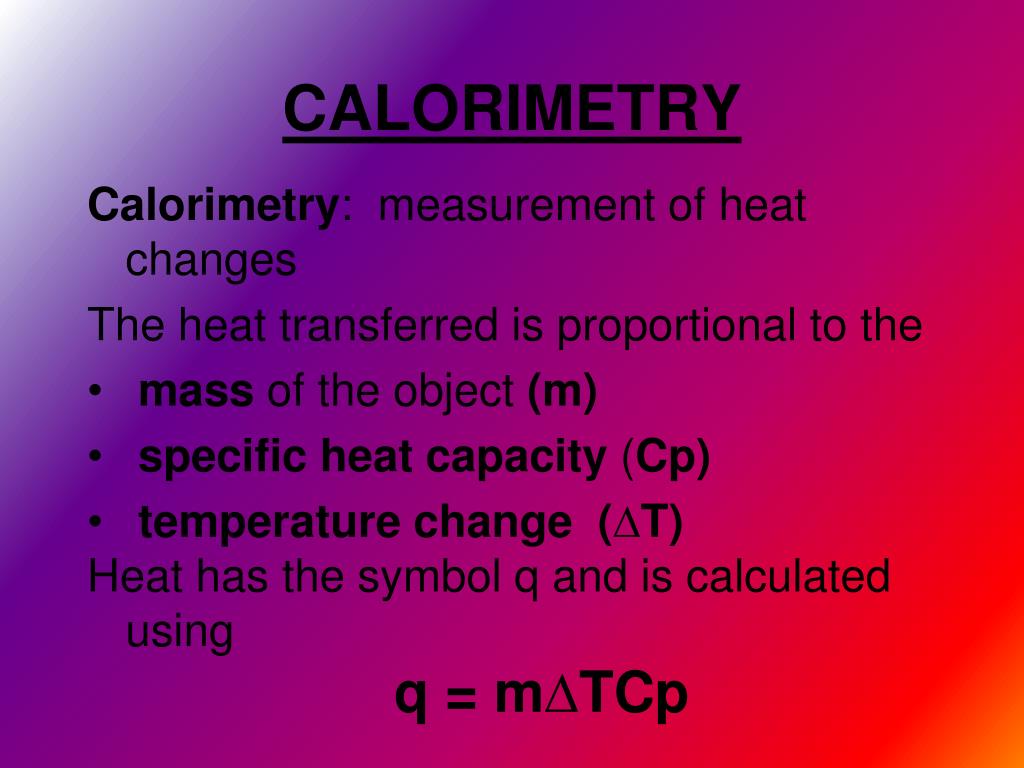
Calorimetry Value Meaning . Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Chemists use calorimetry to determine the amount of heat. How to calculate enthalpy change using calorimetry method. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Energy change, specific heat capacity, temperature of surroundings. Technically, this is called an adiabatic surface in.
from www.slideserve.com
Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Technically, this is called an adiabatic surface in. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. How to calculate enthalpy change using calorimetry method. Energy change, specific heat capacity, temperature of surroundings. Chemists use calorimetry to determine the amount of heat. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction.
PPT CALORIMETRY PowerPoint Presentation, free download ID5767247
Calorimetry Value Meaning Chemists use calorimetry to determine the amount of heat. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Energy change, specific heat capacity, temperature of surroundings. How to calculate enthalpy change using calorimetry method. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Chemists use calorimetry to determine the amount of heat. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. Technically, this is called an adiabatic surface in. Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings.
From www.youtube.com
Calorimetry calculation YouTube Calorimetry Value Meaning How to calculate enthalpy change using calorimetry method. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Chemists use calorimetry to determine the amount of heat. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Technically, this is. Calorimetry Value Meaning.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID1875569 Calorimetry Value Meaning Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. Technically, this is called an adiabatic surface in. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. How to calculate enthalpy change. Calorimetry Value Meaning.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimetry Value Meaning Chemists use calorimetry to determine the amount of heat. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Energy change, specific heat capacity, temperature of surroundings. Calorimetry is a. Calorimetry Value Meaning.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Calorimetry Value Meaning Chemists use calorimetry to determine the amount of heat. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. Calorimetry is a branch of science. Calorimetry Value Meaning.
From www.slideserve.com
PPT CALORIMETRY PowerPoint Presentation, free download ID5767247 Calorimetry Value Meaning A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. How to calculate enthalpy change using calorimetry method. Calorimetry is a field of thermochemistry that measures the amount of heat involved in. Calorimetry Value Meaning.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses Calorimetry Value Meaning Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. Energy change, specific heat capacity, temperature of surroundings. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimetry is. Calorimetry Value Meaning.
From www.slideshare.net
Lesson Enthalpy and Calorimetry Calorimetry Value Meaning Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. How to calculate enthalpy change using calorimetry method. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Energy change, specific heat capacity, temperature of surroundings. Calorimetry is a method of measuring the heat. Calorimetry Value Meaning.
From studylib.net
Calorimetry Calorimetry Value Meaning Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. How. Calorimetry Value Meaning.
From joiikjtqc.blob.core.windows.net
Calculations For Calorimetry at Frank Lewis blog Calorimetry Value Meaning Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. Technically, this is called an adiabatic surface in. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimetry measures. Calorimetry Value Meaning.
From www.youtube.com
050 Calorimetry YouTube Calorimetry Value Meaning Chemists use calorimetry to determine the amount of heat. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Energy change, specific heat capacity, temperature of surroundings. How to calculate enthalpy change using calorimetry method. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. Calorimetry Value Meaning.
From slidetodoc.com
CHEM 1011 Calorimetry The Determination of the Specific Calorimetry Value Meaning How to calculate enthalpy change using calorimetry method. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimetry measures enthalpy changes during chemical processes, where the. Calorimetry Value Meaning.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Calorimetry Value Meaning Chemists use calorimetry to determine the amount of heat. Technically, this is called an adiabatic surface in. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Energy change, specific. Calorimetry Value Meaning.
From www.youtube.com
09.05 Calorimetry YouTube Calorimetry Value Meaning Chemists use calorimetry to determine the amount of heat. How to calculate enthalpy change using calorimetry method. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. Energy change, specific heat capacity,. Calorimetry Value Meaning.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimetry Value Meaning Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Technically, this is called an adiabatic surface in. How to calculate enthalpy change using calorimetry method. A. Calorimetry Value Meaning.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Calorimetry Value Meaning How to calculate enthalpy change using calorimetry method. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter is an instrument that has a thermally isolated compartment. Calorimetry Value Meaning.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimetry Value Meaning Energy change, specific heat capacity, temperature of surroundings. Technically, this is called an adiabatic surface in. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. How to calculate enthalpy change using calorimetry method. Calorimetry is the process of measuring the amount of heat released or absorbed during a. Calorimetry Value Meaning.
From joicpmtos.blob.core.windows.net
Calorimeter In Particle Physics at Krystina Moberg blog Calorimetry Value Meaning Energy change, specific heat capacity, temperature of surroundings. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. Calorimetry is a field of thermochemistry that measures the. Calorimetry Value Meaning.
From www.chemistrystudent.com
Calorimetry (ALevel) ChemistryStudent Calorimetry Value Meaning Chemists use calorimetry to determine the amount of heat. How to calculate enthalpy change using calorimetry method. Energy change, specific heat capacity, temperature of surroundings. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow. Calorimetry Value Meaning.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID373573 Calorimetry Value Meaning A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Technically, this is called an adiabatic surface in. Energy change, specific heat capacity, temperature of surroundings. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. How to calculate enthalpy. Calorimetry Value Meaning.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Calorimetry Value Meaning Energy change, specific heat capacity, temperature of surroundings. Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. How to. Calorimetry Value Meaning.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types Calorimetry Value Meaning Technically, this is called an adiabatic surface in. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. How to calculate enthalpy change using calorimetry method. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is a branch of science concerned with. Calorimetry Value Meaning.
From www.youtube.com
Principle of Calorimetry YouTube Calorimetry Value Meaning Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. How to calculate enthalpy change using calorimetry method. Technically, this is called an adiabatic surface. Calorimetry Value Meaning.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Calorimetry Value Meaning A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. How to calculate enthalpy change using calorimetry method. Technically, this is called an adiabatic surface in. Chemists use calorimetry to. Calorimetry Value Meaning.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Calorimetry Value Meaning Energy change, specific heat capacity, temperature of surroundings. Technically, this is called an adiabatic surface in. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is a branch of science concerned with. Calorimetry Value Meaning.
From loevclgsu.blob.core.windows.net
Calorimeter Thermochemistry Definition at Leona Fraher blog Calorimetry Value Meaning Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Chemists use calorimetry to determine the amount of heat. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. How to calculate enthalpy change using calorimetry method. Technically, this is. Calorimetry Value Meaning.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID1084959 Calorimetry Value Meaning Technically, this is called an adiabatic surface in. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. How to calculate enthalpy change using calorimetry method. A calorimeter is an instrument that has a. Calorimetry Value Meaning.
From faqguide.co
What does a calorimeter do? Explained by FAQGuide Calorimetry Value Meaning Technically, this is called an adiabatic surface in. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Chemists use calorimetry to determine the amount of heat. Energy change, specific heat capacity, temperature of surroundings. Calorimetry is the process of measuring the amount of heat released or absorbed during. Calorimetry Value Meaning.
From courses.lumenlearning.com
Calorimetry Chemistry Atoms First Calorimetry Value Meaning Technically, this is called an adiabatic surface in. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Energy change, specific heat capacity, temperature of surroundings. Chemists use calorimetry to determine the amount of heat. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent. Calorimetry Value Meaning.
From scienceinfo.com
Calorimetry Definition, Principle, Types, Application, and Limitations Calorimetry Value Meaning Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states. Calorimetry Value Meaning.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation Calorimetry Value Meaning Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat. Calorimetry Value Meaning.
From fyohqoqgp.blob.core.windows.net
Calorimeter Formula In Physics at Caroline Graig blog Calorimetry Value Meaning Energy change, specific heat capacity, temperature of surroundings. A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is a method of measuring the heat transfer within a chemical. Calorimetry Value Meaning.
From www.youtube.com
CHEMISTRY 101 Constant Pressure Calorimetry YouTube Calorimetry Value Meaning Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. Technically, this is called an adiabatic surface in. A calorimeter is an instrument that has. Calorimetry Value Meaning.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimetry Value Meaning A calorimeter is an instrument that has a thermally isolated compartment that is designed to prevent heat flow to the surroundings. Chemists use calorimetry to determine the amount of heat. Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. Technically, this is called. Calorimetry Value Meaning.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Calorimetry Value Meaning Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. Technically, this is called an adiabatic surface in. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Energy change,. Calorimetry Value Meaning.
From exoleilmf.blob.core.windows.net
Calorimetric Power Calculation at Martin Stradford blog Calorimetry Value Meaning Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Technically, this is called an adiabatic surface in. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is a method of measuring the heat transfer. Calorimetry Value Meaning.