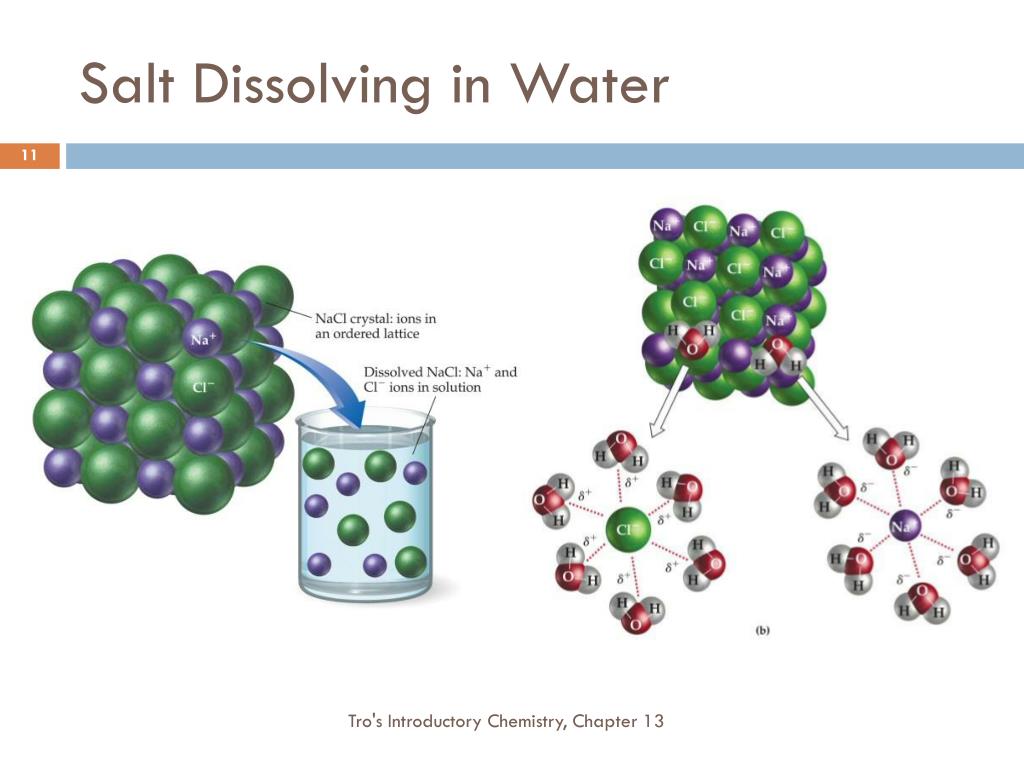
When Table Salt Dissolves In Water . When table salt is dissolved in water, the salt acts as _____ and the water is the solvent. Water typically dissolves many ionic compounds and polar molecules. Nonpolar molecules such as those found in grease or oil do not dissolve in. The water molecules surround the ions and pull them apart, creating. When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly electronegative oxygen. Salt is dissolved into a water solvent. When salt is added to water, the positive and negative ions separate. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. When you dissolve table salt (sodium chloride, also known as nacl) in water, are you producing a chemical change or a physical change? Yes, it can be extracted through evaporation. Well, a chemical change involves a. Can salt be extracted, and will it retain its properties?
from www.slideserve.com
Yes, it can be extracted through evaporation. When you dissolve table salt (sodium chloride, also known as nacl) in water, are you producing a chemical change or a physical change? When salt is added to water, the positive and negative ions separate. When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly electronegative oxygen. Can salt be extracted, and will it retain its properties? We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. Salt is dissolved into a water solvent. When table salt is dissolved in water, the salt acts as _____ and the water is the solvent. Nonpolar molecules such as those found in grease or oil do not dissolve in. Well, a chemical change involves a.
PPT Chapter 13 Solutions PowerPoint Presentation, free download ID
When Table Salt Dissolves In Water When salt is added to water, the positive and negative ions separate. When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly electronegative oxygen. Well, a chemical change involves a. The water molecules surround the ions and pull them apart, creating. When you dissolve table salt (sodium chloride, also known as nacl) in water, are you producing a chemical change or a physical change? Salt is dissolved into a water solvent. When table salt is dissolved in water, the salt acts as _____ and the water is the solvent. Yes, it can be extracted through evaporation. Water typically dissolves many ionic compounds and polar molecules. Nonpolar molecules such as those found in grease or oil do not dissolve in. Can salt be extracted, and will it retain its properties? We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. When salt is added to water, the positive and negative ions separate.
From www.transtutors.com
(Get Answer) Tdoor Therm Meter When Table Salt (NaCl) Dissolves In When Table Salt Dissolves In Water Well, a chemical change involves a. Water typically dissolves many ionic compounds and polar molecules. When salt is added to water, the positive and negative ions separate. When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly electronegative oxygen. Yes, it can be extracted through evaporation. When table salt is dissolved in water,. When Table Salt Dissolves In Water.
From shaunmwilliams.com
Chapter 11 Presentation When Table Salt Dissolves In Water Well, a chemical change involves a. Yes, it can be extracted through evaporation. When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly electronegative oxygen. Water typically dissolves many ionic compounds and polar molecules. When salt is added to water, the positive and negative ions separate. When you dissolve table salt (sodium chloride,. When Table Salt Dissolves In Water.
From www.sliderbase.com
Solutions and solubility Presentation Chemistry When Table Salt Dissolves In Water Salt is dissolved into a water solvent. When table salt is dissolved in water, the salt acts as _____ and the water is the solvent. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. When you dissolve table salt (sodium chloride, also known as nacl) in water, are. When Table Salt Dissolves In Water.
From www.pinterest.com
Is Dissolving Salt in Water a Chemical Change or a Physical Change? When Table Salt Dissolves In Water When you dissolve table salt (sodium chloride, also known as nacl) in water, are you producing a chemical change or a physical change? When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly electronegative oxygen. When table salt is dissolved in water, the salt acts as _____ and the water is the solvent.. When Table Salt Dissolves In Water.
From elchoroukhost.net
Write The Chemical Formulas Of Two Ions When Table Salt Dissolved In When Table Salt Dissolves In Water Yes, it can be extracted through evaporation. Can salt be extracted, and will it retain its properties? Well, a chemical change involves a. When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly electronegative oxygen. Salt is dissolved into a water solvent. When you dissolve table salt (sodium chloride, also known as nacl). When Table Salt Dissolves In Water.
From www.slideserve.com
PPT Chapter 13 Solutions PowerPoint Presentation, free download ID When Table Salt Dissolves In Water Salt is dissolved into a water solvent. When you dissolve table salt (sodium chloride, also known as nacl) in water, are you producing a chemical change or a physical change? Can salt be extracted, and will it retain its properties? Yes, it can be extracted through evaporation. Nonpolar molecules such as those found in grease or oil do not dissolve. When Table Salt Dissolves In Water.
From www.wikidoc.org
Solution wikidoc When Table Salt Dissolves In Water When you dissolve table salt (sodium chloride, also known as nacl) in water, are you producing a chemical change or a physical change? Yes, it can be extracted through evaporation. The water molecules surround the ions and pull them apart, creating. When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly electronegative oxygen.. When Table Salt Dissolves In Water.
From mungfali.com
Dissolving Salt In Water Diagram When Table Salt Dissolves In Water When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly electronegative oxygen. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. When you dissolve table salt (sodium chloride, also known as nacl) in water, are you producing a chemical change or. When Table Salt Dissolves In Water.
From www.youtube.com
Dissolving Salt in Water YouTube When Table Salt Dissolves In Water The water molecules surround the ions and pull them apart, creating. Salt is dissolved into a water solvent. When table salt is dissolved in water, the salt acts as _____ and the water is the solvent. When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly electronegative oxygen. When salt is added to. When Table Salt Dissolves In Water.
From www.youtube.com
table salt dissolves in water YouTube When Table Salt Dissolves In Water Water typically dissolves many ionic compounds and polar molecules. When you dissolve table salt (sodium chloride, also known as nacl) in water, are you producing a chemical change or a physical change? Well, a chemical change involves a. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. Salt. When Table Salt Dissolves In Water.
From www.wikihow.com
How to Dissolve Salt in Water 9 Steps (with Pictures) wikiHow When Table Salt Dissolves In Water When you dissolve table salt (sodium chloride, also known as nacl) in water, are you producing a chemical change or a physical change? Well, a chemical change involves a. Salt is dissolved into a water solvent. Nonpolar molecules such as those found in grease or oil do not dissolve in. Yes, it can be extracted through evaporation. When salt is. When Table Salt Dissolves In Water.
From sciencing.com
What Happens When Salt Is Added to Water? Sciencing When Table Salt Dissolves In Water Yes, it can be extracted through evaporation. When you dissolve table salt (sodium chloride, also known as nacl) in water, are you producing a chemical change or a physical change? Nonpolar molecules such as those found in grease or oil do not dissolve in. Water typically dissolves many ionic compounds and polar molecules. Well, a chemical change involves a. We. When Table Salt Dissolves In Water.
From www.slideserve.com
PPT Chemical Reactions Chapter 7 PowerPoint Presentation, free When Table Salt Dissolves In Water Can salt be extracted, and will it retain its properties? Yes, it can be extracted through evaporation. When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly electronegative oxygen. When salt is added to water, the positive and negative ions separate. When table salt is dissolved in water, the salt acts as _____. When Table Salt Dissolves In Water.
From shaunmwilliams.com
Lecture 11 Presentation When Table Salt Dissolves In Water When you dissolve table salt (sodium chloride, also known as nacl) in water, are you producing a chemical change or a physical change? When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly electronegative oxygen. When salt is added to water, the positive and negative ions separate. Well, a chemical change involves a.. When Table Salt Dissolves In Water.
From www.visionlearning.com
Solutions, Solubility, and Colligative Properties Chemistry When Table Salt Dissolves In Water We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. Water typically dissolves many ionic compounds and polar molecules. When salt is added to water, the positive and negative ions separate. The water molecules surround the ions and pull them apart, creating. Well, a chemical change involves a. Can. When Table Salt Dissolves In Water.
From exonfafiq.blob.core.windows.net
Does Salt Dissolve In Room Temperature Water at Peter Allen blog When Table Salt Dissolves In Water When table salt is dissolved in water, the salt acts as _____ and the water is the solvent. The water molecules surround the ions and pull them apart, creating. Yes, it can be extracted through evaporation. When you dissolve table salt (sodium chloride, also known as nacl) in water, are you producing a chemical change or a physical change? We. When Table Salt Dissolves In Water.
From wou.edu
CH150 Chapter 7 Solutions Chemistry When Table Salt Dissolves In Water We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. When table salt is dissolved in water, the salt acts as _____ and the water is the solvent. Well, a chemical change involves a. Nonpolar molecules such as those found in grease or oil do not dissolve in. Yes,. When Table Salt Dissolves In Water.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free When Table Salt Dissolves In Water When salt is added to water, the positive and negative ions separate. When table salt is dissolved in water, the salt acts as _____ and the water is the solvent. Water typically dissolves many ionic compounds and polar molecules. Yes, it can be extracted through evaporation. Nonpolar molecules such as those found in grease or oil do not dissolve in.. When Table Salt Dissolves In Water.
From awesomehome.co
Table Salt Chemical Formula Equation Awesome Home When Table Salt Dissolves In Water Yes, it can be extracted through evaporation. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. The water molecules surround the ions and pull them apart, creating. When you dissolve table salt (sodium chloride, also known as nacl) in water, are you producing a chemical change or a. When Table Salt Dissolves In Water.
From www.wikihow.com
How to Dissolve Salt in Water 9 Steps (with Pictures) wikiHow When Table Salt Dissolves In Water When you dissolve table salt (sodium chloride, also known as nacl) in water, are you producing a chemical change or a physical change? Well, a chemical change involves a. Water typically dissolves many ionic compounds and polar molecules. When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly electronegative oxygen. Can salt be. When Table Salt Dissolves In Water.
From fphoto.photoshelter.com
science chemistry experiment solubility Fundamental Photographs The When Table Salt Dissolves In Water The water molecules surround the ions and pull them apart, creating. Salt is dissolved into a water solvent. When salt is added to water, the positive and negative ions separate. When table salt is dissolved in water, the salt acts as _____ and the water is the solvent. We will first examine the process that occurs when an ionic compound,. When Table Salt Dissolves In Water.
From stock.adobe.com
Common table salt, sodium chloride (NaCl) and how it dissolves in water When Table Salt Dissolves In Water Well, a chemical change involves a. When table salt is dissolved in water, the salt acts as _____ and the water is the solvent. Nonpolar molecules such as those found in grease or oil do not dissolve in. When salt is added to water, the positive and negative ions separate. We will first examine the process that occurs when an. When Table Salt Dissolves In Water.
From exovcunbz.blob.core.windows.net
Nacl (Table Salt) Dissolves In Water at Ira Canup blog When Table Salt Dissolves In Water When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly electronegative oxygen. Nonpolar molecules such as those found in grease or oil do not dissolve in. Water typically dissolves many ionic compounds and polar molecules. The water molecules surround the ions and pull them apart, creating. When salt is added to water, the. When Table Salt Dissolves In Water.
From www.youtube.com
table salt dissolves in water YouTube When Table Salt Dissolves In Water When you dissolve table salt (sodium chloride, also known as nacl) in water, are you producing a chemical change or a physical change? The water molecules surround the ions and pull them apart, creating. When table salt is dissolved in water, the salt acts as _____ and the water is the solvent. Yes, it can be extracted through evaporation. Can. When Table Salt Dissolves In Water.
From eduvik.in
Matter in Our Surroundings Class 9 Notes Science Chapter 1 Eduvik When Table Salt Dissolves In Water When you dissolve table salt (sodium chloride, also known as nacl) in water, are you producing a chemical change or a physical change? Nonpolar molecules such as those found in grease or oil do not dissolve in. Well, a chemical change involves a. The water molecules surround the ions and pull them apart, creating. We will first examine the process. When Table Salt Dissolves In Water.
From www.youtube.com
Essential Chemistry Dissolving Salt in Water YouTube When Table Salt Dissolves In Water Salt is dissolved into a water solvent. Water typically dissolves many ionic compounds and polar molecules. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly electronegative oxygen. When you dissolve table. When Table Salt Dissolves In Water.
From manualwiringbrancher.z14.web.core.windows.net
Diagram Of Salt Dissolving In Water When Table Salt Dissolves In Water We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. Water typically dissolves many ionic compounds and polar molecules. Yes, it can be extracted through evaporation. Well, a chemical change involves a. When you dissolve table salt (sodium chloride, also known as nacl) in water, are you producing a. When Table Salt Dissolves In Water.
From www.slideserve.com
PPT BASIC’S OF SOLUTION CHEMISTRY PowerPoint Presentation, free When Table Salt Dissolves In Water The water molecules surround the ions and pull them apart, creating. When salt is added to water, the positive and negative ions separate. When table salt is dissolved in water, the salt acts as _____ and the water is the solvent. Water typically dissolves many ionic compounds and polar molecules. Yes, it can be extracted through evaporation. When table salt. When Table Salt Dissolves In Water.
From www.youtube.com
Salt dissolves in water Is this a chemical or physical change? YouTube When Table Salt Dissolves In Water Salt is dissolved into a water solvent. We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. When table salt is dissolved in water, the salt acts as _____ and the water is the solvent. When salt is added to water, the positive and negative ions separate. Water typically. When Table Salt Dissolves In Water.
From www.slideserve.com
PPT Chapter 10 Chemical Reactivity PowerPoint Presentation, free When Table Salt Dissolves In Water Salt is dissolved into a water solvent. Nonpolar molecules such as those found in grease or oil do not dissolve in. Yes, it can be extracted through evaporation. When you dissolve table salt (sodium chloride, also known as nacl) in water, are you producing a chemical change or a physical change? Water typically dissolves many ionic compounds and polar molecules.. When Table Salt Dissolves In Water.
From slidetodoc.com
Salts and Solubility Activity 3 Solution Equilibrium and When Table Salt Dissolves In Water When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly electronegative oxygen. Yes, it can be extracted through evaporation. When table salt is dissolved in water, the salt acts as _____ and the water is the solvent. Salt is dissolved into a water solvent. When you dissolve table salt (sodium chloride, also known. When Table Salt Dissolves In Water.
From www.youtube.com
Table Salt (NaCl) and Its Dissolving in Water Under a Microscope (40x When Table Salt Dissolves In Water When salt is added to water, the positive and negative ions separate. Water typically dissolves many ionic compounds and polar molecules. When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly electronegative oxygen. When you dissolve table salt (sodium chloride, also known as nacl) in water, are you producing a chemical change or. When Table Salt Dissolves In Water.
From slidetodoc.com
Salts and Solubility Activity 3 Solution Equilibrium and When Table Salt Dissolves In Water When table salt is dissolved in water, the salt acts as _____ and the water is the solvent. When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly electronegative oxygen. Nonpolar molecules such as those found in grease or oil do not dissolve in. The water molecules surround the ions and pull them. When Table Salt Dissolves In Water.
From www.youtube.com
How Salt Dissolves in Water? YouTube When Table Salt Dissolves In Water Well, a chemical change involves a. Salt is dissolved into a water solvent. When table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly electronegative oxygen. When table salt is dissolved in water, the salt acts as _____ and the water is the solvent. The water molecules surround the ions and pull them apart,. When Table Salt Dissolves In Water.
From study.com
Solubility of Common Salts What Happens when Salt Dissolves in Water When Table Salt Dissolves In Water We will first examine the process that occurs when an ionic compound, such as table salt (sodium chloride), dissolves in water. Can salt be extracted, and will it retain its properties? When you dissolve table salt (sodium chloride, also known as nacl) in water, are you producing a chemical change or a physical change? Yes, it can be extracted through. When Table Salt Dissolves In Water.