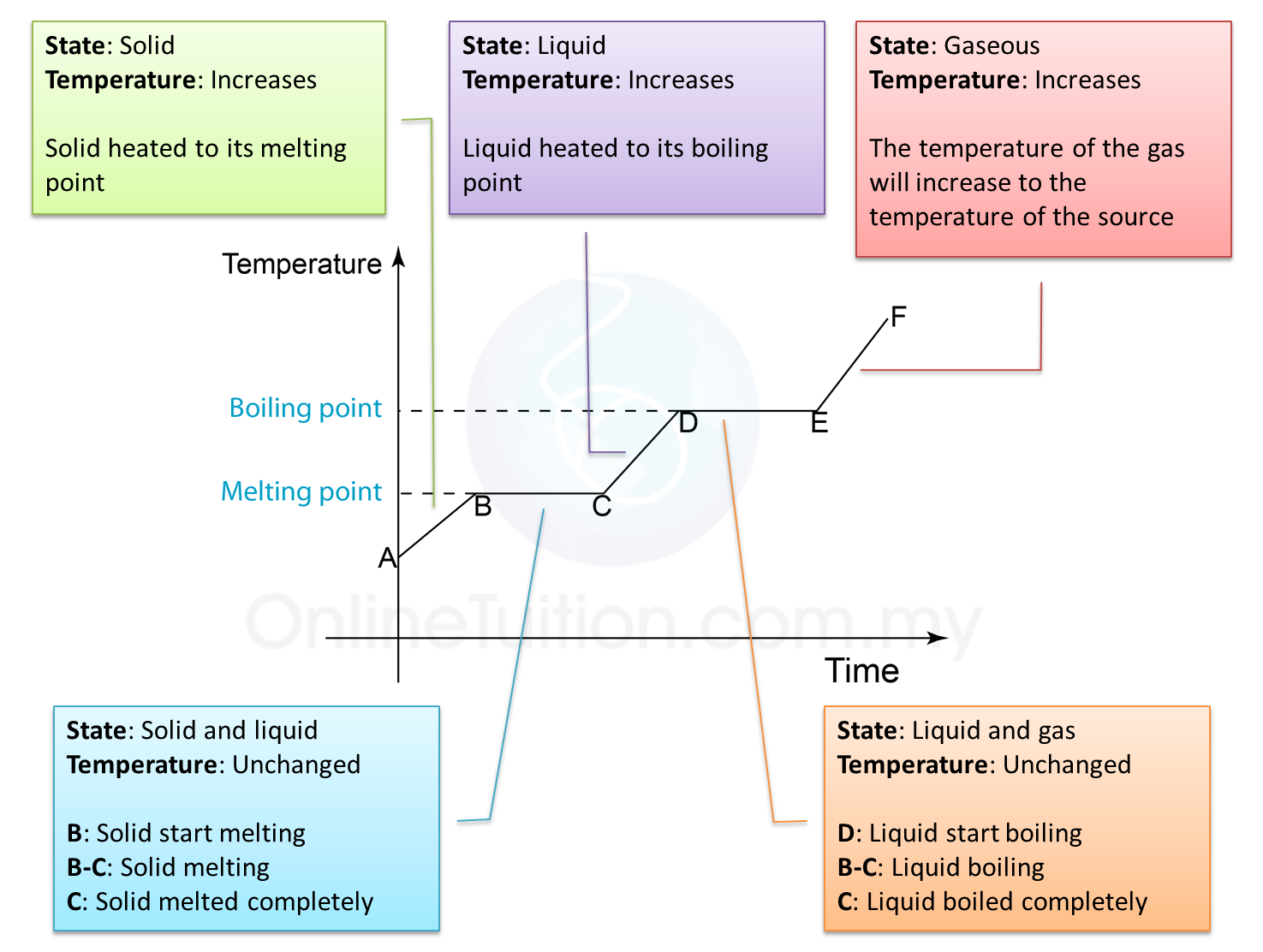
Heating Curve Mcat . If you are moving to the right on the heating curve, the heat values are all positive (heat is being absorbed by the system). 150j/min x 4min = 600j Q = m × c × δ t (see previous chapter on. If you are moving to. The heat needed to change the temperature of a given substance (with no change in phase) is: Heat energy (the total bond energy of reactants or products in a chemical reaction) speeds up the motion of molecules, increasing the. The formula you would use for phase changes is q=mcat. Heating curves show how a substance's temperature changes as heat is added, displaying phase changes such as enthalpy of fusion and enthalpy of vaporization. Heat steam from 100 °c to 120 °c. Heating rate = 150 j/min if the substance takes 4 minutes to melt, how much heat energy was used to melt it? The reason for this is that you can rearrange the formula to solve for.
from spmphysics.onlinetuition.com.my
If you are moving to. Heating rate = 150 j/min if the substance takes 4 minutes to melt, how much heat energy was used to melt it? Heating curves show how a substance's temperature changes as heat is added, displaying phase changes such as enthalpy of fusion and enthalpy of vaporization. Q = m × c × δ t (see previous chapter on. If you are moving to the right on the heating curve, the heat values are all positive (heat is being absorbed by the system). Heat steam from 100 °c to 120 °c. 150j/min x 4min = 600j The heat needed to change the temperature of a given substance (with no change in phase) is: The reason for this is that you can rearrange the formula to solve for. Heat energy (the total bond energy of reactants or products in a chemical reaction) speeds up the motion of molecules, increasing the.
The Heating Curve SPM Physics Form 4/Form 5 Revision Notes
Heating Curve Mcat Heat steam from 100 °c to 120 °c. Heat steam from 100 °c to 120 °c. The formula you would use for phase changes is q=mcat. Heating rate = 150 j/min if the substance takes 4 minutes to melt, how much heat energy was used to melt it? The heat needed to change the temperature of a given substance (with no change in phase) is: 150j/min x 4min = 600j Heat energy (the total bond energy of reactants or products in a chemical reaction) speeds up the motion of molecules, increasing the. The reason for this is that you can rearrange the formula to solve for. If you are moving to. If you are moving to the right on the heating curve, the heat values are all positive (heat is being absorbed by the system). Heating curves show how a substance's temperature changes as heat is added, displaying phase changes such as enthalpy of fusion and enthalpy of vaporization. Q = m × c × δ t (see previous chapter on.
From www.numerade.com
SOLVEDUse the following heating curve for water to answer the Heating Curve Mcat Heat energy (the total bond energy of reactants or products in a chemical reaction) speeds up the motion of molecules, increasing the. The heat needed to change the temperature of a given substance (with no change in phase) is: 150j/min x 4min = 600j If you are moving to. Heating rate = 150 j/min if the substance takes 4 minutes. Heating Curve Mcat.
From www.youtube.com
Heating Curves Tutorial How to Calculate enthalpy changes in Heating Heating Curve Mcat If you are moving to the right on the heating curve, the heat values are all positive (heat is being absorbed by the system). Heating rate = 150 j/min if the substance takes 4 minutes to melt, how much heat energy was used to melt it? The formula you would use for phase changes is q=mcat. 150j/min x 4min =. Heating Curve Mcat.
From jackwestin.com
Phase Diagram Pressure And Temperature Energy Changes In Chemical Heating Curve Mcat Heating rate = 150 j/min if the substance takes 4 minutes to melt, how much heat energy was used to melt it? The formula you would use for phase changes is q=mcat. The reason for this is that you can rearrange the formula to solve for. Heating curves show how a substance's temperature changes as heat is added, displaying phase. Heating Curve Mcat.
From bceweb.org
Heating Curve Chart A Visual Reference of Charts Chart Master Heating Curve Mcat The formula you would use for phase changes is q=mcat. Heating rate = 150 j/min if the substance takes 4 minutes to melt, how much heat energy was used to melt it? If you are moving to. If you are moving to the right on the heating curve, the heat values are all positive (heat is being absorbed by the. Heating Curve Mcat.
From www.worldwisetutoring.com
Heating and Cooling Curves Heating Curve Mcat If you are moving to. Q = m × c × δ t (see previous chapter on. The reason for this is that you can rearrange the formula to solve for. Heat energy (the total bond energy of reactants or products in a chemical reaction) speeds up the motion of molecules, increasing the. Heating rate = 150 j/min if the. Heating Curve Mcat.
From slideplayer.com
Heating and Cooling Curves ppt download Heating Curve Mcat The formula you would use for phase changes is q=mcat. The heat needed to change the temperature of a given substance (with no change in phase) is: Heating curves show how a substance's temperature changes as heat is added, displaying phase changes such as enthalpy of fusion and enthalpy of vaporization. 150j/min x 4min = 600j If you are moving. Heating Curve Mcat.
From schoolbag.info
Systems and Processes Thermochemistry Training MCAT General Heating Curve Mcat Q = m × c × δ t (see previous chapter on. 150j/min x 4min = 600j If you are moving to the right on the heating curve, the heat values are all positive (heat is being absorbed by the system). Heating curves show how a substance's temperature changes as heat is added, displaying phase changes such as enthalpy of. Heating Curve Mcat.
From www.youtube.com
HTPIB14B Specific Heat and Q = mcT YouTube Heating Curve Mcat The reason for this is that you can rearrange the formula to solve for. Heating curves show how a substance's temperature changes as heat is added, displaying phase changes such as enthalpy of fusion and enthalpy of vaporization. If you are moving to the right on the heating curve, the heat values are all positive (heat is being absorbed by. Heating Curve Mcat.
From www.slideserve.com
PPT THE STRUCTURE OF THE ATOM PowerPoint Presentation, free download Heating Curve Mcat Heat steam from 100 °c to 120 °c. The heat needed to change the temperature of a given substance (with no change in phase) is: 150j/min x 4min = 600j Heating curves show how a substance's temperature changes as heat is added, displaying phase changes such as enthalpy of fusion and enthalpy of vaporization. If you are moving to the. Heating Curve Mcat.
From www.numerade.com
SOLVED A plateau (horizontal line) on a heating or cooling curve Heating Curve Mcat If you are moving to the right on the heating curve, the heat values are all positive (heat is being absorbed by the system). Heating curves show how a substance's temperature changes as heat is added, displaying phase changes such as enthalpy of fusion and enthalpy of vaporization. Heat steam from 100 °c to 120 °c. The heat needed to. Heating Curve Mcat.
From www.youtube.com
How to read and interpret heatingcooling curve YouTube Heating Curve Mcat Heating curves show how a substance's temperature changes as heat is added, displaying phase changes such as enthalpy of fusion and enthalpy of vaporization. Heating rate = 150 j/min if the substance takes 4 minutes to melt, how much heat energy was used to melt it? The heat needed to change the temperature of a given substance (with no change. Heating Curve Mcat.
From slideplayer.com
Heating Curves and Phase Diagrams ppt download Heating Curve Mcat Q = m × c × δ t (see previous chapter on. Heating rate = 150 j/min if the substance takes 4 minutes to melt, how much heat energy was used to melt it? Heat steam from 100 °c to 120 °c. Heat energy (the total bond energy of reactants or products in a chemical reaction) speeds up the motion. Heating Curve Mcat.
From www.slideserve.com
PPT Unit 3 Energy and States PowerPoint Presentation, free download Heating Curve Mcat If you are moving to the right on the heating curve, the heat values are all positive (heat is being absorbed by the system). The heat needed to change the temperature of a given substance (with no change in phase) is: Heating rate = 150 j/min if the substance takes 4 minutes to melt, how much heat energy was used. Heating Curve Mcat.
From ch301.cm.utexas.edu
heating curve Heating Curve Mcat 150j/min x 4min = 600j Heating curves show how a substance's temperature changes as heat is added, displaying phase changes such as enthalpy of fusion and enthalpy of vaporization. Heating rate = 150 j/min if the substance takes 4 minutes to melt, how much heat energy was used to melt it? Heat steam from 100 °c to 120 °c. The. Heating Curve Mcat.
From www.ck12.org
Heating and Cooling Curves CK12 Foundation Heating Curve Mcat Heating rate = 150 j/min if the substance takes 4 minutes to melt, how much heat energy was used to melt it? Heat steam from 100 °c to 120 °c. If you are moving to. Heating curves show how a substance's temperature changes as heat is added, displaying phase changes such as enthalpy of fusion and enthalpy of vaporization. Q. Heating Curve Mcat.
From www.youtube.com
Heating Curve Discussion YouTube Heating Curve Mcat Heat steam from 100 °c to 120 °c. Heating rate = 150 j/min if the substance takes 4 minutes to melt, how much heat energy was used to melt it? The formula you would use for phase changes is q=mcat. The heat needed to change the temperature of a given substance (with no change in phase) is: The reason for. Heating Curve Mcat.
From slideplayer.com
Heating Curves and Phase Diagrams ppt download Heating Curve Mcat Heating rate = 150 j/min if the substance takes 4 minutes to melt, how much heat energy was used to melt it? If you are moving to the right on the heating curve, the heat values are all positive (heat is being absorbed by the system). The heat needed to change the temperature of a given substance (with no change. Heating Curve Mcat.
From www.youtube.com
Class 11 Physics Calorimetry 14 Heating Curve For NEET and JEE Heating Curve Mcat Heating rate = 150 j/min if the substance takes 4 minutes to melt, how much heat energy was used to melt it? The formula you would use for phase changes is q=mcat. 150j/min x 4min = 600j Heating curves show how a substance's temperature changes as heat is added, displaying phase changes such as enthalpy of fusion and enthalpy of. Heating Curve Mcat.
From www.youtube.com
How to Read a Heating Curve YouTube Heating Curve Mcat Heat energy (the total bond energy of reactants or products in a chemical reaction) speeds up the motion of molecules, increasing the. The formula you would use for phase changes is q=mcat. The reason for this is that you can rearrange the formula to solve for. 150j/min x 4min = 600j Heat steam from 100 °c to 120 °c. Heating. Heating Curve Mcat.
From evulpo.com
Heating and cooling curves Science Explanation & Exercises evulpo Heating Curve Mcat Heating curves show how a substance's temperature changes as heat is added, displaying phase changes such as enthalpy of fusion and enthalpy of vaporization. If you are moving to the right on the heating curve, the heat values are all positive (heat is being absorbed by the system). Heat energy (the total bond energy of reactants or products in a. Heating Curve Mcat.
From www.expii.com
Heating and Cooling Curves — Overview & Examples Expii Heating Curve Mcat Heat steam from 100 °c to 120 °c. The formula you would use for phase changes is q=mcat. If you are moving to the right on the heating curve, the heat values are all positive (heat is being absorbed by the system). If you are moving to. Heating rate = 150 j/min if the substance takes 4 minutes to melt,. Heating Curve Mcat.
From spmphysics.onlinetuition.com.my
The Heating Curve SPM Physics Form 4/Form 5 Revision Notes Heating Curve Mcat Heating rate = 150 j/min if the substance takes 4 minutes to melt, how much heat energy was used to melt it? If you are moving to. The formula you would use for phase changes is q=mcat. The reason for this is that you can rearrange the formula to solve for. If you are moving to the right on the. Heating Curve Mcat.
From www.youtube.com
HEATING CURVE How to Read & How TO Draw A Heating Curve [ AboodyTV Heating Curve Mcat 150j/min x 4min = 600j The formula you would use for phase changes is q=mcat. Heat energy (the total bond energy of reactants or products in a chemical reaction) speeds up the motion of molecules, increasing the. If you are moving to the right on the heating curve, the heat values are all positive (heat is being absorbed by the. Heating Curve Mcat.
From wordwall.net
Heating curve Labelled diagram Heating Curve Mcat If you are moving to the right on the heating curve, the heat values are all positive (heat is being absorbed by the system). Heat energy (the total bond energy of reactants or products in a chemical reaction) speeds up the motion of molecules, increasing the. Q = m × c × δ t (see previous chapter on. Heating curves. Heating Curve Mcat.
From jackwestin.com
Phase Diagram Pressure And Temperature Energy Changes In Chemical Heating Curve Mcat The formula you would use for phase changes is q=mcat. Q = m × c × δ t (see previous chapter on. Heat steam from 100 °c to 120 °c. Heating rate = 150 j/min if the substance takes 4 minutes to melt, how much heat energy was used to melt it? Heat energy (the total bond energy of reactants. Heating Curve Mcat.
From slideplayer.com
The Heating Curve Mr. Shields Regents Chemistry U07 L ppt download Heating Curve Mcat The reason for this is that you can rearrange the formula to solve for. If you are moving to. Q = m × c × δ t (see previous chapter on. The heat needed to change the temperature of a given substance (with no change in phase) is: If you are moving to the right on the heating curve, the. Heating Curve Mcat.
From schematicdiagramglocer.z19.web.core.windows.net
Heating Curve Chemistry Diagram Heating Curve Mcat Heat energy (the total bond energy of reactants or products in a chemical reaction) speeds up the motion of molecules, increasing the. Heat steam from 100 °c to 120 °c. The reason for this is that you can rearrange the formula to solve for. Heating rate = 150 j/min if the substance takes 4 minutes to melt, how much heat. Heating Curve Mcat.
From www.slideserve.com
PPT Phase Diagrams & Heating Curves PowerPoint Presentation, free Heating Curve Mcat Heating rate = 150 j/min if the substance takes 4 minutes to melt, how much heat energy was used to melt it? The heat needed to change the temperature of a given substance (with no change in phase) is: Heat steam from 100 °c to 120 °c. If you are moving to. Q = m × c × δ t. Heating Curve Mcat.
From www.albert.io
Heating Curve and Specific Heat Chemistry Practice Albert Heating Curve Mcat The formula you would use for phase changes is q=mcat. Heat energy (the total bond energy of reactants or products in a chemical reaction) speeds up the motion of molecules, increasing the. Heating rate = 150 j/min if the substance takes 4 minutes to melt, how much heat energy was used to melt it? 150j/min x 4min = 600j Heat. Heating Curve Mcat.
From www.youtube.com
OAT H2O Heating Curve YouTube Heating Curve Mcat Heat energy (the total bond energy of reactants or products in a chemical reaction) speeds up the motion of molecules, increasing the. Q = m × c × δ t (see previous chapter on. The formula you would use for phase changes is q=mcat. Heating curves show how a substance's temperature changes as heat is added, displaying phase changes such. Heating Curve Mcat.
From circuitdiagramalexandra.z5.web.core.windows.net
Heating Curve Diagram Heating Curve Mcat Heat steam from 100 °c to 120 °c. The formula you would use for phase changes is q=mcat. Heating curves show how a substance's temperature changes as heat is added, displaying phase changes such as enthalpy of fusion and enthalpy of vaporization. The reason for this is that you can rearrange the formula to solve for. The heat needed to. Heating Curve Mcat.
From www.youtube.com
Heating and Cooling Curve / Introduction plus and Potential Heating Curve Mcat The heat needed to change the temperature of a given substance (with no change in phase) is: The reason for this is that you can rearrange the formula to solve for. Q = m × c × δ t (see previous chapter on. Heating curves show how a substance's temperature changes as heat is added, displaying phase changes such as. Heating Curve Mcat.
From app.jove.com
Heating and Cooling Curves Concept Chemistry JoVe Heating Curve Mcat Heating curves show how a substance's temperature changes as heat is added, displaying phase changes such as enthalpy of fusion and enthalpy of vaporization. The heat needed to change the temperature of a given substance (with no change in phase) is: Q = m × c × δ t (see previous chapter on. Heat steam from 100 °c to 120. Heating Curve Mcat.
From schoolbag.info
Figure 7.7. Heating Curve for a Single Compound Heating Curve Mcat The formula you would use for phase changes is q=mcat. If you are moving to. Heat steam from 100 °c to 120 °c. Heating curves show how a substance's temperature changes as heat is added, displaying phase changes such as enthalpy of fusion and enthalpy of vaporization. Heating rate = 150 j/min if the substance takes 4 minutes to melt,. Heating Curve Mcat.
From 2023.igem.wiki
Overview Heating Curve Mcat If you are moving to the right on the heating curve, the heat values are all positive (heat is being absorbed by the system). Heat steam from 100 °c to 120 °c. The formula you would use for phase changes is q=mcat. Heat energy (the total bond energy of reactants or products in a chemical reaction) speeds up the motion. Heating Curve Mcat.