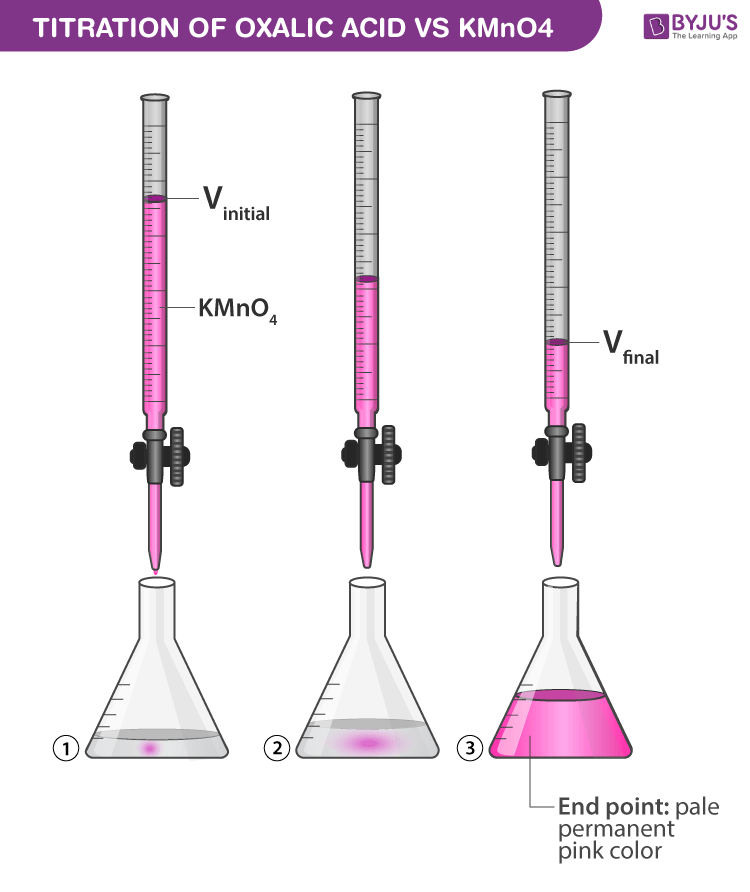
Redox Titration Using Kmno4 . In close proximity to the endpoint, the action of the indicator is analogous to the. Our goal is to sketch the titration curve quickly, using as few. Use our revision notes to understand how a redox titration in a level chemistry can be used to calculate the percentage mass of an element in a compound. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. Iron (ii) with potassium permanganate. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Iron (ii) ions are titrated with potassium permanganate (kmno 4) in an acidic medium. In this section we demonstrate a simple method for sketching a redox titration curve. The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. Titrate with kmno4 at 60~90 oc with stirring. The titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. 30 s) pink color as the end point.
from byjus.com
In close proximity to the endpoint, the action of the indicator is analogous to the. Iron (ii) with potassium permanganate. Our goal is to sketch the titration curve quickly, using as few. Use our revision notes to understand how a redox titration in a level chemistry can be used to calculate the percentage mass of an element in a compound. Titrate with kmno4 at 60~90 oc with stirring. The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. 30 s) pink color as the end point. The titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration.
Titration of Oxalic Acid with KMnO4 Chemistry Practicals Class 12
Redox Titration Using Kmno4 The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. 30 s) pink color as the end point. The titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. Titrate with kmno4 at 60~90 oc with stirring. Iron (ii) with potassium permanganate. Iron (ii) ions are titrated with potassium permanganate (kmno 4) in an acidic medium. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Our goal is to sketch the titration curve quickly, using as few. In this section we demonstrate a simple method for sketching a redox titration curve. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. In close proximity to the endpoint, the action of the indicator is analogous to the. Use our revision notes to understand how a redox titration in a level chemistry can be used to calculate the percentage mass of an element in a compound.
From www.chegg.com
Solved 4. In this experiment; as you perform redox titration Redox Titration Using Kmno4 In this section we demonstrate a simple method for sketching a redox titration curve. Our goal is to sketch the titration curve quickly, using as few. The titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such. Redox Titration Using Kmno4.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Redox Titration Using Kmno4 The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Titrate with kmno4 at 60~90 oc with stirring. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. 30 s) pink color. Redox Titration Using Kmno4.
From www.numerade.com
SOLVEDRedox Titrationi Acidic Medium Puch Slider Upto Add Volume of Redox Titration Using Kmno4 Our goal is to sketch the titration curve quickly, using as few. 30 s) pink color as the end point. The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. Use our revision notes to understand how a redox titration in a level chemistry can be used to calculate. Redox Titration Using Kmno4.
From www.youtube.com
Redox Titration II KMnO4 vs Oxalic acid II Self indicator YouTube Redox Titration Using Kmno4 Use our revision notes to understand how a redox titration in a level chemistry can be used to calculate the percentage mass of an element in a compound. In this section we demonstrate a simple method for sketching a redox titration curve. In close proximity to the endpoint, the action of the indicator is analogous to the. The titration of. Redox Titration Using Kmno4.
From www.thesciencehive.co.uk
Redox and Electrode Potentials — the science hive Redox Titration Using Kmno4 Our goal is to sketch the titration curve quickly, using as few. Iron (ii) with potassium permanganate. Iron (ii) ions are titrated with potassium permanganate (kmno 4) in an acidic medium. The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. The titration of potassium permanganate (kmno 4) against. Redox Titration Using Kmno4.
From www.youtube.com
Titration of KMnO4 vs Ferrous ammonium sulphate Redox titrationHow to Redox Titration Using Kmno4 Iron (ii) with potassium permanganate. Use our revision notes to understand how a redox titration in a level chemistry can be used to calculate the percentage mass of an element in a compound. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. In close proximity to the. Redox Titration Using Kmno4.
From www.slideserve.com
PPT Ch 16 Redox Titrations PowerPoint Presentation, free download Redox Titration Using Kmno4 In this section we demonstrate a simple method for sketching a redox titration curve. Iron (ii) with potassium permanganate. Our goal is to sketch the titration curve quickly, using as few. In close proximity to the endpoint, the action of the indicator is analogous to the. In this experiment you will use a standard solution of potassium permanganate (kmno 4). Redox Titration Using Kmno4.
From www.numerade.com
SOLVED redox titrations Calculate the mass of the original FeCl2 Redox Titration Using Kmno4 In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. In close proximity to the endpoint, the action of the indicator is analogous to the. Iron (ii) ions are titrated with potassium permanganate (kmno 4) in an acidic medium. 30 s) pink color as. Redox Titration Using Kmno4.
From byjus.com
Titration of Oxalic Acid with KMnO4 Chemistry Practicals Class 12 Redox Titration Using Kmno4 In close proximity to the endpoint, the action of the indicator is analogous to the. 30 s) pink color as the end point. The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. The titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration.. Redox Titration Using Kmno4.
From chemistryfromscratch.org
V2.10 Redox Titration Using Kmno4 30 s) pink color as the end point. Titrate with kmno4 at 60~90 oc with stirring. Use our revision notes to understand how a redox titration in a level chemistry can be used to calculate the percentage mass of an element in a compound. In this section we demonstrate a simple method for sketching a redox titration curve. Our goal. Redox Titration Using Kmno4.
From slideplayer.com
Unit 12 (Chp 20) Electrochemistry (E, ∆G, K) ppt download Redox Titration Using Kmno4 Iron (ii) ions are titrated with potassium permanganate (kmno 4) in an acidic medium. In close proximity to the endpoint, the action of the indicator is analogous to the. 30 s) pink color as the end point. In this section we demonstrate a simple method for sketching a redox titration curve. Titrate with kmno4 at 60~90 oc with stirring. Our. Redox Titration Using Kmno4.
From www.youtube.com
Determination of Concentration of KMnO4 Soution using Ferrous Ammonium Redox Titration Using Kmno4 30 s) pink color as the end point. In this section we demonstrate a simple method for sketching a redox titration curve. The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. Titrate with kmno4 at 60~90 oc with stirring. The titration of potassium permanganate (kmno 4) against mohr. Redox Titration Using Kmno4.
From byjus.com
One mole of KMnO4 is used for complete oxidation of FeSO4, FeC2O4 and Redox Titration Using Kmno4 Iron (ii) ions are titrated with potassium permanganate (kmno 4) in an acidic medium. In close proximity to the endpoint, the action of the indicator is analogous to the. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. Iron (ii) with potassium permanganate.. Redox Titration Using Kmno4.
From www.youtube.com
General Chemistry Standardization of KMnO4 By Redox Titration YouTube Redox Titration Using Kmno4 The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. The titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. 30 s) pink color as the end point. Iron (ii) ions are titrated with potassium permanganate (kmno 4) in an acidic medium. Our. Redox Titration Using Kmno4.
From quizlet.com
Diagram REDOX Titration 1 To prepare a standard solution of Ammonium Redox Titration Using Kmno4 Titrate with kmno4 at 60~90 oc with stirring. The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. 30 s) pink color as the end point. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Iron. Redox Titration Using Kmno4.
From www.numerade.com
SOLVED MnO4 + H2O2 > Mn2+ + O2 The above reaction was used for a Redox Titration Using Kmno4 The titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. Use our revision notes to understand how a redox titration in a level chemistry can be used to calculate the percentage mass of. Redox Titration Using Kmno4.
From www.numerade.com
SOLVED Potassium permanganate (KMnO4) was used as an oxidizing titrant Redox Titration Using Kmno4 The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. The titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. Use. Redox Titration Using Kmno4.
From www.youtube.com
class 12 PRACTICAL CHEMISTRY Redox Titration of KMnO4 against Mohr's Redox Titration Using Kmno4 Iron (ii) ions are titrated with potassium permanganate (kmno 4) in an acidic medium. In close proximity to the endpoint, the action of the indicator is analogous to the. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Use our revision notes to understand how a redox. Redox Titration Using Kmno4.
From www.slideserve.com
PPT REDOX TITRATION PowerPoint Presentation, free download ID431911 Redox Titration Using Kmno4 Iron (ii) with potassium permanganate. In this section we demonstrate a simple method for sketching a redox titration curve. Iron (ii) ions are titrated with potassium permanganate (kmno 4) in an acidic medium. Use our revision notes to understand how a redox titration in a level chemistry can be used to calculate the percentage mass of an element in a. Redox Titration Using Kmno4.
From www.studypool.com
SOLUTION determine the percentage of ferrous sulphate solution using Redox Titration Using Kmno4 The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Iron (ii) with potassium permanganate. In this section we demonstrate a simple method for sketching a redox titration curve. Iron (ii) ions are titrated with potassium permanganate (kmno 4) in an acidic medium. Use our revision notes to. Redox Titration Using Kmno4.
From www.youtube.com
Standardization of KMnO4 Redox Titration KMnO4 Vs Fe2+ Mohrs salt Redox Titration Using Kmno4 Use our revision notes to understand how a redox titration in a level chemistry can be used to calculate the percentage mass of an element in a compound. The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. The titration of potassium permanganate (kmno 4) against mohr salt is. Redox Titration Using Kmno4.
From studylib.net
Experiment 8 Redox Titrations Potassium permanganate, KMnO4 Redox Titration Using Kmno4 In close proximity to the endpoint, the action of the indicator is analogous to the. Iron (ii) ions are titrated with potassium permanganate (kmno 4) in an acidic medium. Iron (ii) with potassium permanganate. In this section we demonstrate a simple method for sketching a redox titration curve. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2. Redox Titration Using Kmno4.
From www.chegg.com
Solved Nazl204 KMnO4 Ex. 2 Titration of 0.2121 g of pure Redox Titration Using Kmno4 30 s) pink color as the end point. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. Use our revision notes to understand how a redox titration in a level chemistry can be used to calculate the percentage mass of an element in. Redox Titration Using Kmno4.
From www.numerade.com
SOLVED Potassium permanganate, KMnO4, is a powerful oxidizing agent Redox Titration Using Kmno4 Iron (ii) ions are titrated with potassium permanganate (kmno 4) in an acidic medium. Iron (ii) with potassium permanganate. Use our revision notes to understand how a redox titration in a level chemistry can be used to calculate the percentage mass of an element in a compound. In close proximity to the endpoint, the action of the indicator is analogous. Redox Titration Using Kmno4.
From www.numerade.com
SOLVED Redox Titration Potassium permanganate, KMnO4, is a strong Redox Titration Using Kmno4 Iron (ii) ions are titrated with potassium permanganate (kmno 4) in an acidic medium. Use our revision notes to understand how a redox titration in a level chemistry can be used to calculate the percentage mass of an element in a compound. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an. Redox Titration Using Kmno4.
From www.youtube.com
Redox Titration Example Question 2 (Easy) ALevel Chemistry YouTube Redox Titration Using Kmno4 Our goal is to sketch the titration curve quickly, using as few. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. Titrate with kmno4 at 60~90 oc with stirring. In this section we demonstrate a simple method for sketching a redox titration curve.. Redox Titration Using Kmno4.
From www.youtube.com
Redox Titration of FeSO4 with KMnO4 and Calculation of Equivalance Redox Titration Using Kmno4 Titrate with kmno4 at 60~90 oc with stirring. 30 s) pink color as the end point. The titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. In this. Redox Titration Using Kmno4.
From www.youtube.com
Balancing redox reaction by Ion electron method KMnO4 and SnCl2 /redox Redox Titration Using Kmno4 Titrate with kmno4 at 60~90 oc with stirring. Use our revision notes to understand how a redox titration in a level chemistry can be used to calculate the percentage mass of an element in a compound. Iron (ii) with potassium permanganate. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example. Redox Titration Using Kmno4.
From www.oceanproperty.co.th
Experiment Redox Titrations Potassium Permanganate,, 41 OFF Redox Titration Using Kmno4 Iron (ii) ions are titrated with potassium permanganate (kmno 4) in an acidic medium. Titrate with kmno4 at 60~90 oc with stirring. 30 s) pink color as the end point. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. In close proximity to. Redox Titration Using Kmno4.
From www.hanlin.com
Edexcel A Level Chemistry复习笔记8.2.1 Redox Titration翰林国际教育 Redox Titration Using Kmno4 30 s) pink color as the end point. In close proximity to the endpoint, the action of the indicator is analogous to the. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. Iron (ii) with potassium permanganate. The titration of potassium permanganate (kmno. Redox Titration Using Kmno4.
From www.youtube.com
KMnO4 Redox Titration With FeSO4.7H20 YouTube Redox Titration Using Kmno4 In this section we demonstrate a simple method for sketching a redox titration curve. 30 s) pink color as the end point. Iron (ii) ions are titrated with potassium permanganate (kmno 4) in an acidic medium. In close proximity to the endpoint, the action of the indicator is analogous to the. Iron (ii) with potassium permanganate. The titration of potassium. Redox Titration Using Kmno4.
From www.studypool.com
SOLUTION Redox titration Studypool Redox Titration Using Kmno4 The redox titration of permanganate ions is commonly used to determine the iron content of a sample, such as iron ores. In this section we demonstrate a simple method for sketching a redox titration curve. Titrate with kmno4 at 60~90 oc with stirring. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the. Redox Titration Using Kmno4.
From chemistrypubs.com
Titration of Oxalic Acid with KMnO4 Chemistrupubs Redox Titration Using Kmno4 Use our revision notes to understand how a redox titration in a level chemistry can be used to calculate the percentage mass of an element in a compound. The titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. Titrate with kmno4 at 60~90 oc with stirring. The titration of potassium permanganate (kmno 4) against. Redox Titration Using Kmno4.
From www.oceanproperty.co.th
Experiment Redox Titrations Potassium Permanganate,, 41 OFF Redox Titration Using Kmno4 In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. The titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an. Redox Titration Using Kmno4.
From www.numerade.com
SOLVED A redox titration using a molar solution of potassium Redox Titration Using Kmno4 The titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. 30 s) pink color as the end point. Iron (ii) with potassium permanganate. Iron (ii) ions are titrated. Redox Titration Using Kmno4.