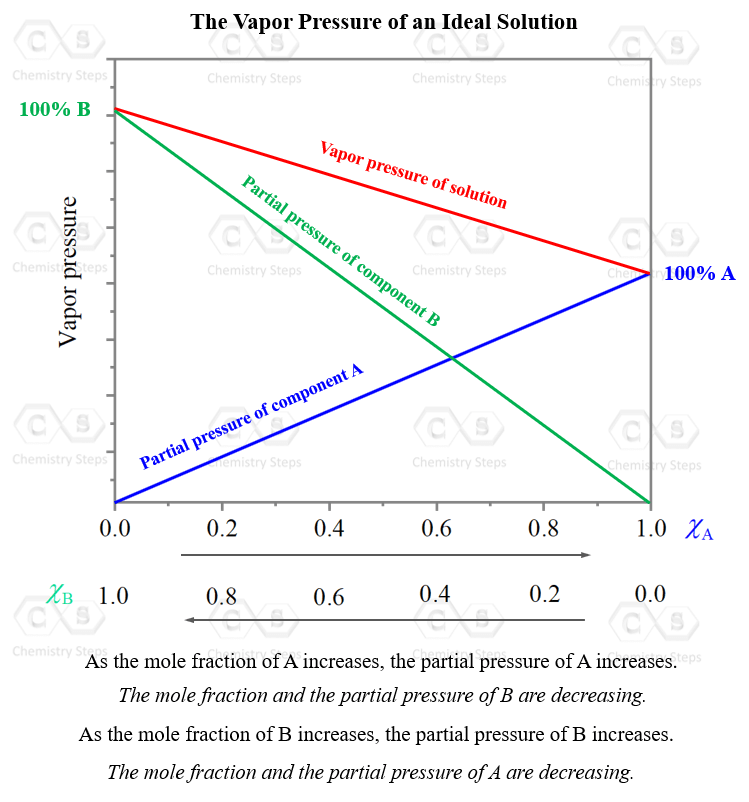
What Is A Vapor Pressure Curve . Vapour pressure is the pressure exerted by a vapour with its condensed phases in a closed system at a given temperature. Learn how vapor pressure curves illustrate the boiling points of liquids and how they are affected by external pressure. Learn how vapour pressure depends on. See examples of how to use the clausius. Learn the definition, characteristics and applications of vapor pressure, the pressure of a vapor in equilibrium with its condensed phases. Learn how vapor pressure curves illustrate the boiling points of liquids and how they are affected by external pressure. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample.
from general.chemistrysteps.com
That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Learn how vapor pressure curves illustrate the boiling points of liquids and how they are affected by external pressure. Learn how vapour pressure depends on. Learn how vapor pressure curves illustrate the boiling points of liquids and how they are affected by external pressure. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Vapour pressure is the pressure exerted by a vapour with its condensed phases in a closed system at a given temperature. See examples of how to use the clausius. Learn the definition, characteristics and applications of vapor pressure, the pressure of a vapor in equilibrium with its condensed phases.
Vapor Pressure Lowering Chemistry Steps
What Is A Vapor Pressure Curve That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Learn how vapor pressure curves illustrate the boiling points of liquids and how they are affected by external pressure. Vapour pressure is the pressure exerted by a vapour with its condensed phases in a closed system at a given temperature. See examples of how to use the clausius. Learn the definition, characteristics and applications of vapor pressure, the pressure of a vapor in equilibrium with its condensed phases. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Learn how vapor pressure curves illustrate the boiling points of liquids and how they are affected by external pressure. Learn how vapour pressure depends on.
From chem.libretexts.org
Chapter 11.4 Vapor Pressure Chemistry LibreTexts What Is A Vapor Pressure Curve That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Learn how vapor pressure curves illustrate the boiling points of liquids and how they are affected by external pressure. Learn the definition, characteristics and applications of vapor pressure, the pressure of a vapor in equilibrium with its condensed phases. Vapour pressure is. What Is A Vapor Pressure Curve.
From www.youtube.com
Lecture 11how to create/Generate Vapor pressure Curve in hysys What Is A Vapor Pressure Curve Learn how vapour pressure depends on. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Learn how vapor pressure curves illustrate the boiling points of liquids and how they are affected by external pressure. Learn the definition, characteristics and applications of vapor pressure, the pressure of a vapor in equilibrium with. What Is A Vapor Pressure Curve.
From www.researchgate.net
Vapour pressure curve (solid line), critical point (black circle) and What Is A Vapor Pressure Curve See examples of how to use the clausius. Learn how vapor pressure curves illustrate the boiling points of liquids and how they are affected by external pressure. Learn how vapour pressure depends on. Learn the definition, characteristics and applications of vapor pressure, the pressure of a vapor in equilibrium with its condensed phases. The vapor pressure of a liquid is. What Is A Vapor Pressure Curve.
From pressbooks.bccampus.ca
2.3 Phase diagrams Introduction to Engineering Thermodynamics What Is A Vapor Pressure Curve Learn the definition, characteristics and applications of vapor pressure, the pressure of a vapor in equilibrium with its condensed phases. Learn how vapour pressure depends on. See examples of how to use the clausius. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Vapour pressure is the pressure exerted by a. What Is A Vapor Pressure Curve.
From studylib.net
Vapor Pressure Curves What Is A Vapor Pressure Curve Learn how vapor pressure curves illustrate the boiling points of liquids and how they are affected by external pressure. Vapour pressure is the pressure exerted by a vapour with its condensed phases in a closed system at a given temperature. Learn how vapour pressure depends on. Learn how vapor pressure curves illustrate the boiling points of liquids and how they. What Is A Vapor Pressure Curve.
From www.slideserve.com
PPT Vapor Pressure and Boiling PowerPoint Presentation, free download What Is A Vapor Pressure Curve See examples of how to use the clausius. Learn how vapour pressure depends on. Vapour pressure is the pressure exerted by a vapour with its condensed phases in a closed system at a given temperature. Learn how vapor pressure curves illustrate the boiling points of liquids and how they are affected by external pressure. Learn how vapor pressure curves illustrate. What Is A Vapor Pressure Curve.
From www.studyblue.com
Chapter 15 Properties of Solids at Yavapai College StudyBlue What Is A Vapor Pressure Curve The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Learn how vapor pressure curves illustrate the boiling points of liquids and how they are affected by external pressure. Vapour pressure is the pressure. What Is A Vapor Pressure Curve.
From courses.lumenlearning.com
Liquid to Gas Phase Transition Introduction to Chemistry What Is A Vapor Pressure Curve Learn how vapor pressure curves illustrate the boiling points of liquids and how they are affected by external pressure. Vapour pressure is the pressure exerted by a vapour with its condensed phases in a closed system at a given temperature. Learn how vapour pressure depends on. Learn how vapor pressure curves illustrate the boiling points of liquids and how they. What Is A Vapor Pressure Curve.
From www.researchgate.net
Vapor pressures of Si, SiO, SiO2, and other metal or metal oxide What Is A Vapor Pressure Curve See examples of how to use the clausius. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Learn how vapour pressure depends on. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Learn how vapor pressure curves illustrate the boiling points of. What Is A Vapor Pressure Curve.
From vacaero.com
Simple Physics for the High Vacuum Processing Industry What Is A Vapor Pressure Curve See examples of how to use the clausius. Learn the definition, characteristics and applications of vapor pressure, the pressure of a vapor in equilibrium with its condensed phases. Learn how vapor pressure curves illustrate the boiling points of liquids and how they are affected by external pressure. Learn how vapor pressure curves illustrate the boiling points of liquids and how. What Is A Vapor Pressure Curve.
From www.researchgate.net
21 Density and Reid Vapor Pressure Curves versus Hydrocracked Naphtha What Is A Vapor Pressure Curve Learn how vapor pressure curves illustrate the boiling points of liquids and how they are affected by external pressure. Vapour pressure is the pressure exerted by a vapour with its condensed phases in a closed system at a given temperature. See examples of how to use the clausius. The vapor pressure of a liquid is the equilibrium pressure of a. What Is A Vapor Pressure Curve.
From vacaero.com
Partial Pressure Brazing What Is A Vapor Pressure Curve Learn how vapor pressure curves illustrate the boiling points of liquids and how they are affected by external pressure. Learn how vapour pressure depends on. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Learn how vapor pressure curves illustrate the boiling points of liquids and how they are affected by. What Is A Vapor Pressure Curve.
From socratic.org
Explain how each of the following affects the vapour pressure of a What Is A Vapor Pressure Curve That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Learn the definition, characteristics and applications of vapor pressure, the pressure of a vapor in equilibrium with its condensed phases. Learn how vapor pressure curves illustrate the boiling points of liquids and how they are affected by external pressure. The vapor pressure. What Is A Vapor Pressure Curve.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps What Is A Vapor Pressure Curve Vapour pressure is the pressure exerted by a vapour with its condensed phases in a closed system at a given temperature. See examples of how to use the clausius. Learn how vapor pressure curves illustrate the boiling points of liquids and how they are affected by external pressure. Learn the definition, characteristics and applications of vapor pressure, the pressure of. What Is A Vapor Pressure Curve.
From www.researchgate.net
Vapor pressure of sulfur allotropes at temperatures over solid sulfur What Is A Vapor Pressure Curve Learn how vapour pressure depends on. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Learn the definition, characteristics and applications of vapor pressure, the pressure of a vapor in equilibrium with its condensed phases. Learn how vapor pressure curves illustrate the boiling points of liquids and how they are affected. What Is A Vapor Pressure Curve.
From www.chegg.com
Solved The vapor pressure curves for methanol, water and What Is A Vapor Pressure Curve The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Learn how vapor pressure curves illustrate the boiling points of liquids and how they are affected by external pressure. Learn the definition, characteristics and applications of vapor pressure, the pressure of a vapor in equilibrium with its condensed phases. That is, the. What Is A Vapor Pressure Curve.
From quizizz.com
Vapor Pressure and Intermolecular forces Quizizz What Is A Vapor Pressure Curve Learn how vapor pressure curves illustrate the boiling points of liquids and how they are affected by external pressure. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Learn the definition, characteristics and applications of vapor pressure, the pressure of a vapor in equilibrium with its condensed phases. Vapour pressure is. What Is A Vapor Pressure Curve.
From www.peoi.org
Chapter 10 Section C Properties of Liquids What Is A Vapor Pressure Curve See examples of how to use the clausius. Learn how vapor pressure curves illustrate the boiling points of liquids and how they are affected by external pressure. Learn how vapour pressure depends on. Learn how vapor pressure curves illustrate the boiling points of liquids and how they are affected by external pressure. That is, the pressure of the vapor resulting. What Is A Vapor Pressure Curve.
From www.chegg.com
Solved Use the vapor pressure curves illustrated here to What Is A Vapor Pressure Curve Learn the definition, characteristics and applications of vapor pressure, the pressure of a vapor in equilibrium with its condensed phases. Vapour pressure is the pressure exerted by a vapour with its condensed phases in a closed system at a given temperature. See examples of how to use the clausius. That is, the pressure of the vapor resulting from evaporation of. What Is A Vapor Pressure Curve.
From chart-studio.plotly.com
Vapor Pressure Curves for Water and Propane scatter chart made by What Is A Vapor Pressure Curve The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Learn how vapour pressure depends on. Learn the definition, characteristics and applications of vapor pressure, the pressure of a vapor in equilibrium with its condensed phases. Learn how vapor pressure curves illustrate the boiling points of liquids and how they are affected. What Is A Vapor Pressure Curve.
From www.chegg.com
Solved (4pts) The graph below is the vapor pressures of What Is A Vapor Pressure Curve Learn how vapor pressure curves illustrate the boiling points of liquids and how they are affected by external pressure. Vapour pressure is the pressure exerted by a vapour with its condensed phases in a closed system at a given temperature. See examples of how to use the clausius. Learn how vapour pressure depends on. That is, the pressure of the. What Is A Vapor Pressure Curve.
From chart-studio.plotly.com
Vapor Pressure Curves for Propane and Water line chart made by What Is A Vapor Pressure Curve The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); See examples of how to use the clausius. Learn the definition, characteristics and applications of vapor pressure, the pressure of a vapor in equilibrium with its condensed phases. Learn how vapor pressure curves illustrate the boiling points of liquids and how they. What Is A Vapor Pressure Curve.
From www.numerade.com
SOLVEDThis portion of a phase diagram shows the vaporpressure curves What Is A Vapor Pressure Curve The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Learn how vapour pressure depends on. Learn how vapor pressure curves illustrate the boiling points of liquids and how they are affected by external. What Is A Vapor Pressure Curve.
From www.numerade.com
SOLVEDUse the vapor pressure curves shown in the figure below for What Is A Vapor Pressure Curve Learn the definition, characteristics and applications of vapor pressure, the pressure of a vapor in equilibrium with its condensed phases. See examples of how to use the clausius. Learn how vapor pressure curves illustrate the boiling points of liquids and how they are affected by external pressure. Learn how vapour pressure depends on. The vapor pressure of a liquid is. What Is A Vapor Pressure Curve.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID1025977 What Is A Vapor Pressure Curve Learn how vapor pressure curves illustrate the boiling points of liquids and how they are affected by external pressure. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Learn how vapor pressure curves illustrate the boiling points of liquids and how they are affected by external pressure. See examples of how. What Is A Vapor Pressure Curve.
From www.researchgate.net
Methanol vapor pressure curve. Markers located at atmospheric pressure What Is A Vapor Pressure Curve Learn how vapor pressure curves illustrate the boiling points of liquids and how they are affected by external pressure. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Learn how vapour pressure depends on. Vapour pressure is the pressure exerted by a vapour with its condensed phases in a closed system. What Is A Vapor Pressure Curve.
From www.chegg.com
Solved Consider the vapor pressure curves for H2O and CCl4 What Is A Vapor Pressure Curve Learn how vapor pressure curves illustrate the boiling points of liquids and how they are affected by external pressure. Learn how vapor pressure curves illustrate the boiling points of liquids and how they are affected by external pressure. Vapour pressure is the pressure exerted by a vapour with its condensed phases in a closed system at a given temperature. That. What Is A Vapor Pressure Curve.
From www.researchgate.net
Oxygen, nitrogen and argon vapor pressure curves. Download Scientific What Is A Vapor Pressure Curve Learn how vapor pressure curves illustrate the boiling points of liquids and how they are affected by external pressure. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Learn the definition, characteristics and applications of vapor pressure, the pressure of a vapor in equilibrium with its condensed phases. See examples of. What Is A Vapor Pressure Curve.
From www.shutterstock.com
Saturated Water Vapor Pressure Curve Marked Stock Vector (Royalty Free What Is A Vapor Pressure Curve See examples of how to use the clausius. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Learn how vapour pressure depends on. Learn the definition, characteristics and applications of vapor pressure, the pressure of a vapor in equilibrium with its condensed phases. Learn how vapor pressure curves illustrate the boiling. What Is A Vapor Pressure Curve.
From www.powerstream.com
Vapor pressures of the Chemical Elements, vapor pressure of metals and What Is A Vapor Pressure Curve See examples of how to use the clausius. Learn how vapour pressure depends on. Learn how vapor pressure curves illustrate the boiling points of liquids and how they are affected by external pressure. Learn the definition, characteristics and applications of vapor pressure, the pressure of a vapor in equilibrium with its condensed phases. That is, the pressure of the vapor. What Is A Vapor Pressure Curve.
From dhilreefer.blogspot.com
Vapor Pressure Curves For Vacuum Brazing What Is A Vapor Pressure Curve The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); See examples of how to use the clausius. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Learn how vapor pressure curves illustrate the boiling points of liquids and how they are affected. What Is A Vapor Pressure Curve.
From www.vrogue.co
Solved Definitions Partial Pressure Of The Water Vapo vrogue.co What Is A Vapor Pressure Curve Learn how vapor pressure curves illustrate the boiling points of liquids and how they are affected by external pressure. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Learn how vapor pressure curves illustrate the boiling points of liquids and how they are affected by external pressure. Learn how vapour pressure. What Is A Vapor Pressure Curve.
From jmpcoblog.com
How To Read A Pump Curve Part 2 What Is A Vapor Pressure Curve Learn the definition, characteristics and applications of vapor pressure, the pressure of a vapor in equilibrium with its condensed phases. See examples of how to use the clausius. That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. Vapour pressure is the pressure exerted by a vapour with its condensed phases in. What Is A Vapor Pressure Curve.
From www.coursehero.com
[Solved] The vapor pressure curves for acetone, ethanol and butanol are What Is A Vapor Pressure Curve Learn how vapour pressure depends on. Vapour pressure is the pressure exerted by a vapour with its condensed phases in a closed system at a given temperature. See examples of how to use the clausius. The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); Learn the definition, characteristics and applications of. What Is A Vapor Pressure Curve.
From www.barth-maschinenbau.de
Operating principle Barth Maschinenbau What Is A Vapor Pressure Curve That is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample. See examples of how to use the clausius. Learn the definition, characteristics and applications of vapor pressure, the pressure of a vapor in equilibrium with its condensed phases. Learn how vapour pressure depends on. Vapour pressure is the pressure exerted by a. What Is A Vapor Pressure Curve.