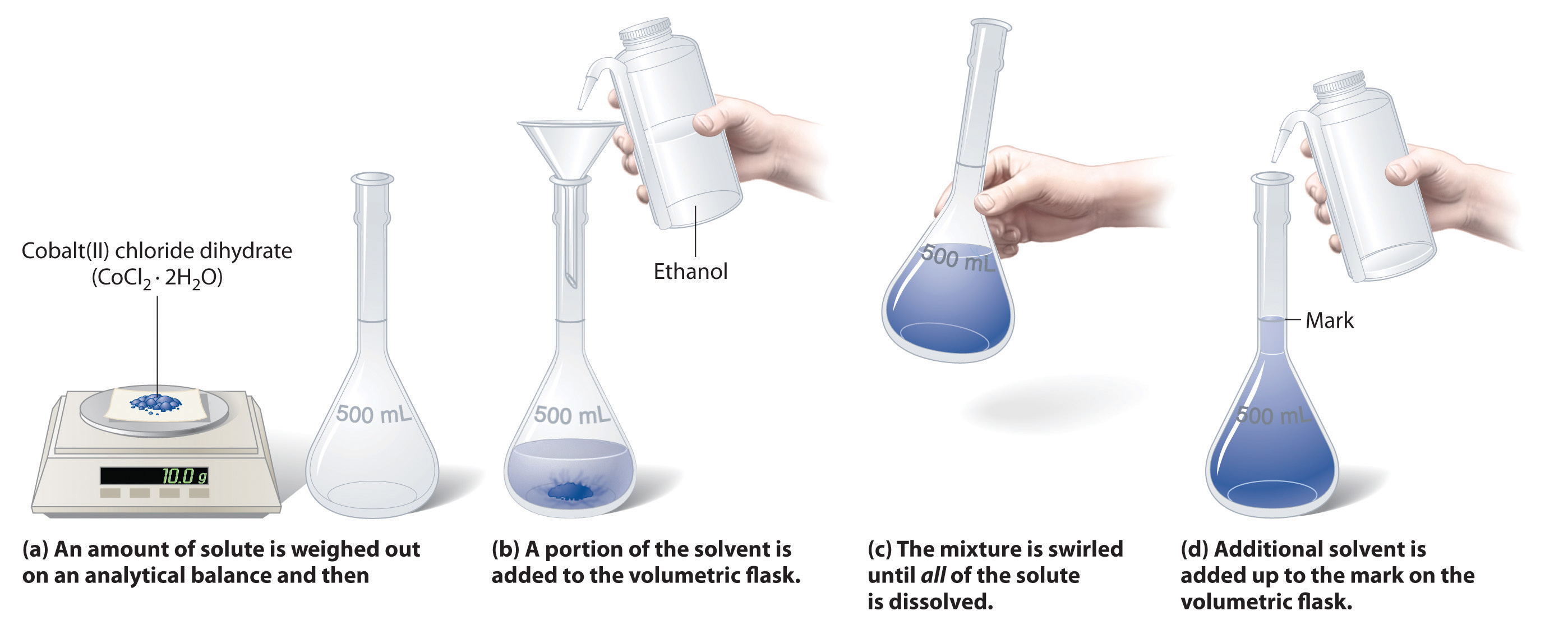
Dilute Solutions Examples . What volume of 1.59 m koh is required to prepare. Volume of a concentrated solution needed for dilution. Dilution of solutions — overview & examples sometimes we need to dilute solutions to a lower concentration. This video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. To dilute solutions, such as simple household solutions, make sure you know the. Use the formula c1v1 = c2v2 to calculate the correct concentration and. Dilution is the addition of. Learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute.
from chem.libretexts.org
This video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. Dilution is the addition of. Learn how to dilute and concentrate solutions. Dilution of solutions — overview & examples sometimes we need to dilute solutions to a lower concentration. To dilute solutions, such as simple household solutions, make sure you know the. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Use the formula c1v1 = c2v2 to calculate the correct concentration and. What volume of 1.59 m koh is required to prepare. Volume of a concentrated solution needed for dilution.
4.3 Solution Concentrations Chemistry LibreTexts
Dilute Solutions Examples Learn how to dilute and concentrate solutions. What volume of 1.59 m koh is required to prepare. Dilution of solutions — overview & examples sometimes we need to dilute solutions to a lower concentration. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Volume of a concentrated solution needed for dilution. Use the formula c1v1 = c2v2 to calculate the correct concentration and. Learn how to dilute and concentrate solutions. To dilute solutions, such as simple household solutions, make sure you know the. This video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. Dilution is the addition of. Often, a worker will need to change the concentration of a solution by changing the amount of solvent.
From www.askiitians.com
Types Of Solutions Study Material for IIT JEE askIITians Dilute Solutions Examples Learn how to dilute and concentrate solutions. Dilution is the addition of. Use the formula c1v1 = c2v2 to calculate the correct concentration and. To dilute solutions, such as simple household solutions, make sure you know the. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. A dilution is a process. Dilute Solutions Examples.
From www.shutterstock.com
228 Dilute Solution Concentrated Solution Images, Stock Photos Dilute Solutions Examples Often, a worker will need to change the concentration of a solution by changing the amount of solvent. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Use the formula c1v1 = c2v2 to calculate the correct concentration and. Learn how to dilute and concentrate solutions.. Dilute Solutions Examples.
From www.yaclass.in
Types of solutions Concentrated and dilute solutions — lesson. Science Dilute Solutions Examples To dilute solutions, such as simple household solutions, make sure you know the. This video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. Dilution is the addition of. Use the formula c1v1 = c2v2 to calculate the correct concentration and. A dilution is a process where the concentration of. Dilute Solutions Examples.
From stock.adobe.com
Solubility vector illustration. Labeled solute, solvent and solution Dilute Solutions Examples This video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. To dilute solutions, such as simple household solutions, make sure you know the. What volume of 1.59 m koh is required to prepare. Use the formula c1v1 = c2v2 to calculate the correct concentration and. Learn how to dilute. Dilute Solutions Examples.
From www.youtube.com
CHEMISTRY 101 Solution Dilutions YouTube Dilute Solutions Examples A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. This video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. To dilute solutions, such as simple household solutions, make sure you know the. Dilution is the. Dilute Solutions Examples.
From fphoto.photoshelter.com
science chemistry solubility dilution Fundamental Photographs The Dilute Solutions Examples Volume of a concentrated solution needed for dilution. Dilution of solutions — overview & examples sometimes we need to dilute solutions to a lower concentration. What volume of 1.59 m koh is required to prepare. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Learn how to dilute and concentrate solutions.. Dilute Solutions Examples.
From www.slideserve.com
PPT Solutions Part II PowerPoint Presentation, free download ID4272422 Dilute Solutions Examples Volume of a concentrated solution needed for dilution. Use the formula c1v1 = c2v2 to calculate the correct concentration and. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. To dilute solutions, such as simple household solutions, make sure you know the. Dilution of solutions — overview & examples sometimes we. Dilute Solutions Examples.
From www.youtube.com
pH of Dilute Solution (Example) YouTube Dilute Solutions Examples What volume of 1.59 m koh is required to prepare. Volume of a concentrated solution needed for dilution. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Use the formula c1v1 = c2v2 to calculate the correct concentration and. A dilution is a process where the concentration of a solution is. Dilute Solutions Examples.
From chem.libretexts.org
4.3 Solution Concentrations Chemistry LibreTexts Dilute Solutions Examples Often, a worker will need to change the concentration of a solution by changing the amount of solvent. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Dilution is the addition of. Dilution of solutions — overview & examples sometimes we need to dilute solutions to. Dilute Solutions Examples.
From www.youtube.com
How to Dilute a Solution YouTube Dilute Solutions Examples To dilute solutions, such as simple household solutions, make sure you know the. Use the formula c1v1 = c2v2 to calculate the correct concentration and. Volume of a concentrated solution needed for dilution. What volume of 1.59 m koh is required to prepare. A dilution is a process where the concentration of a solution is lowered by adding solvent to. Dilute Solutions Examples.
From www.thoughtco.com
Dilution Calculations From Stock Solutions in Chemistry Dilute Solutions Examples To dilute solutions, such as simple household solutions, make sure you know the. Volume of a concentrated solution needed for dilution. Use the formula c1v1 = c2v2 to calculate the correct concentration and. This video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. Dilution of solutions — overview &. Dilute Solutions Examples.
From www.youtube.com
Chem 11 Dilution Calculations_Solving for Final Volume after Dilution Dilute Solutions Examples Learn how to dilute and concentrate solutions. Dilution of solutions — overview & examples sometimes we need to dilute solutions to a lower concentration. Volume of a concentrated solution needed for dilution. Use the formula c1v1 = c2v2 to calculate the correct concentration and. Often, a worker will need to change the concentration of a solution by changing the amount. Dilute Solutions Examples.
From www.slideserve.com
PPT Diluting Solutions PowerPoint Presentation, free download ID Dilute Solutions Examples Dilution of solutions — overview & examples sometimes we need to dilute solutions to a lower concentration. To dilute solutions, such as simple household solutions, make sure you know the. This video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. What volume of 1.59 m koh is required to. Dilute Solutions Examples.
From www.youtube.com
Aqueous, Dilute And Concentrated Solution, General Science Lecture Dilute Solutions Examples A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Volume of a concentrated solution needed for dilution. What volume of 1.59 m koh is required to prepare. Dilution of solutions — overview & examples sometimes we need to dilute solutions to a lower concentration. This video. Dilute Solutions Examples.
From www.youtube.com
What is Dilute Solution? Chemistry YouTube Dilute Solutions Examples A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Learn how to dilute and concentrate solutions. Dilution is the addition of. To dilute solutions, such as simple household solutions, make sure you know the. Often, a worker will need to change the concentration of a solution. Dilute Solutions Examples.
From www.wikihow.com
How to Dilute Solutions 8 Steps (with Pictures) wikiHow Dilute Solutions Examples Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Volume of a concentrated solution needed for dilution. Use the formula c1v1 = c2v2 to calculate the correct concentration and. What volume of 1.59 m koh is required to prepare. This video and written tutorial will guide you through how to make. Dilute Solutions Examples.
From general.chemistrysteps.com
Dilution of a Stock Solution and Calculations Based Morality Dilute Solutions Examples Volume of a concentrated solution needed for dilution. What volume of 1.59 m koh is required to prepare. Dilution of solutions — overview & examples sometimes we need to dilute solutions to a lower concentration. Use the formula c1v1 = c2v2 to calculate the correct concentration and. This video and written tutorial will guide you through how to make a. Dilute Solutions Examples.
From studylib.net
Diluting a Solution Dilute Solutions Examples A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Dilution is the addition of. Use the formula c1v1 = c2v2 to calculate the correct concentration and. This video and written tutorial will guide you through how to make a dilution and how to calculate the dilution. Dilute Solutions Examples.
From www.pinterest.cl
Dilution when solvent is added to dilute a solution, the number of Dilute Solutions Examples To dilute solutions, such as simple household solutions, make sure you know the. Dilution of solutions — overview & examples sometimes we need to dilute solutions to a lower concentration. This video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. Dilution is the addition of. Use the formula c1v1. Dilute Solutions Examples.
From www.pinterest.com
Dilute Flashcards, Easy science, Science student Dilute Solutions Examples To dilute solutions, such as simple household solutions, make sure you know the. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Use the formula c1v1 = c2v2 to calculate the correct concentration and. This video and written tutorial will guide you through how to make a dilution and how to. Dilute Solutions Examples.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilute Solutions Examples This video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. To dilute solutions, such as simple household solutions, make sure you know the. Dilution is the addition of. What volume of 1.59 m koh is required to prepare. Often, a worker will need to change the concentration of a. Dilute Solutions Examples.
From www.yaclass.in
Types of solutions Concentrated and dilute solutions — lesson. Science Dilute Solutions Examples To dilute solutions, such as simple household solutions, make sure you know the. Use the formula c1v1 = c2v2 to calculate the correct concentration and. Volume of a concentrated solution needed for dilution. This video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. Learn how to dilute and concentrate. Dilute Solutions Examples.
From slidetodoc.com
WATER AND SOLUTIONS A solution is a mixture Dilute Solutions Examples Learn how to dilute and concentrate solutions. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. What volume of 1.59 m koh is required to prepare. Dilution is the addition of. This video and written tutorial will guide you through how to make a dilution and. Dilute Solutions Examples.
From www.youtube.com
Dilute and Concentrated Solution YouTube Dilute Solutions Examples To dilute solutions, such as simple household solutions, make sure you know the. What volume of 1.59 m koh is required to prepare. Dilution is the addition of. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Dilution of solutions — overview & examples sometimes we need to dilute solutions to. Dilute Solutions Examples.
From www.youtube.com
Dilution formula and how to use it to solve problems. 3 examples solved Dilute Solutions Examples To dilute solutions, such as simple household solutions, make sure you know the. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Learn how to dilute and concentrate. Dilute Solutions Examples.
From fphoto.photoshelter.com
science chemistry solubility dilution Fundamental Photographs The Dilute Solutions Examples Use the formula c1v1 = c2v2 to calculate the correct concentration and. What volume of 1.59 m koh is required to prepare. This video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. Volume of a concentrated solution needed for dilution. A dilution is a process where the concentration of. Dilute Solutions Examples.
From chem.libretexts.org
5.2 Solutions and Dilutions Chemistry LibreTexts Dilute Solutions Examples Dilution is the addition of. What volume of 1.59 m koh is required to prepare. This video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Dilution. Dilute Solutions Examples.
From www.slideserve.com
PPT Chapter 15 Solutions PowerPoint Presentation, free download ID Dilute Solutions Examples Volume of a concentrated solution needed for dilution. Dilution is the addition of. What volume of 1.59 m koh is required to prepare. To dilute solutions, such as simple household solutions, make sure you know the. Learn how to dilute and concentrate solutions. Use the formula c1v1 = c2v2 to calculate the correct concentration and. This video and written tutorial. Dilute Solutions Examples.
From www.youtube.com
Chem121 Dilution Equation 8 5 YouTube Dilute Solutions Examples Volume of a concentrated solution needed for dilution. Learn how to dilute and concentrate solutions. To dilute solutions, such as simple household solutions, make sure you know the. A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. Dilution is the addition of. Use the formula c1v1. Dilute Solutions Examples.
From study.com
Dilution Definition, Equation & Factors Video & Lesson Transcript Dilute Solutions Examples What volume of 1.59 m koh is required to prepare. To dilute solutions, such as simple household solutions, make sure you know the. Learn how to dilute and concentrate solutions. Dilution of solutions — overview & examples sometimes we need to dilute solutions to a lower concentration. This video and written tutorial will guide you through how to make a. Dilute Solutions Examples.
From fphoto.photoshelter.com
science chemistry solubility dilution Fundamental Photographs The Dilute Solutions Examples Often, a worker will need to change the concentration of a solution by changing the amount of solvent. Dilution of solutions — overview & examples sometimes we need to dilute solutions to a lower concentration. Volume of a concentrated solution needed for dilution. Dilution is the addition of. What volume of 1.59 m koh is required to prepare. This video. Dilute Solutions Examples.
From www.youtube.com
Diluting a 12 M stock solution YouTube Dilute Solutions Examples Volume of a concentrated solution needed for dilution. Learn how to dilute and concentrate solutions. Dilution of solutions — overview & examples sometimes we need to dilute solutions to a lower concentration. Dilution is the addition of. Often, a worker will need to change the concentration of a solution by changing the amount of solvent. This video and written tutorial. Dilute Solutions Examples.
From www.toppr.com
Dilution Formula Definition, Concepts and Examples Dilute Solutions Examples Use the formula c1v1 = c2v2 to calculate the correct concentration and. Dilution of solutions — overview & examples sometimes we need to dilute solutions to a lower concentration. Dilution is the addition of. To dilute solutions, such as simple household solutions, make sure you know the. A dilution is a process where the concentration of a solution is lowered. Dilute Solutions Examples.
From www.wikihow.com
How to Dilute Solutions 8 Steps (with Pictures) wikiHow Dilute Solutions Examples Use the formula c1v1 = c2v2 to calculate the correct concentration and. To dilute solutions, such as simple household solutions, make sure you know the. What volume of 1.59 m koh is required to prepare. Learn how to dilute and concentrate solutions. Volume of a concentrated solution needed for dilution. A dilution is a process where the concentration of a. Dilute Solutions Examples.
From www.slideshare.net
Water and solutions Dilute Solutions Examples A dilution is a process where the concentration of a solution is lowered by adding solvent to the solution without adding more solute. This video and written tutorial will guide you through how to make a dilution and how to calculate the dilution factor. Dilution is the addition of. Volume of a concentrated solution needed for dilution. Learn how to. Dilute Solutions Examples.