Iron (Iii) Chloride React With Potassium Iodide . A solution of chlorine can displace iodine from potassium iodide solution: First answer (by the chemist) is wrong; Chlorine + potassium iodide → potassium chloride + iodine. Chlorine + potassium iodide → iodine + potassium chloride: A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. The reaction between chlorine and iron (ii) ions. Iron ions as a catalyst in the reaction between persulphate ions and iodide ions. Cl 2 + 2ki → 2kcl + i 2. Chlorine reacts with potassium to form the solid, potassium chloride. Write the balanced symbol equation for this reaction, including state. Cl 2 (g) + 2ki(aq) → i 2 (aq) + 2kcl(aq) The equation for the reaction between chlorine and potassium iodide is shown below. Identify which species has been:. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the. We described a precipitation reaction in which a colorless solution of.
from www.numerade.com
Cl 2 + 2ki → 2kcl + i 2. A solution of chlorine can displace iodine from potassium iodide solution: Iron ions as a catalyst in the reaction between persulphate ions and iodide ions. We described a precipitation reaction in which a colorless solution of. The reaction between chlorine and iron (ii) ions. Fei 3 does not exist, this is a redox reaction, i 2 (or its complex ki 3 with ki) is the. A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. Chlorine + potassium iodide → iodine + potassium chloride: The equation for the reaction between chlorine and potassium iodide is shown below. Write the balanced symbol equation for this reaction, including state.
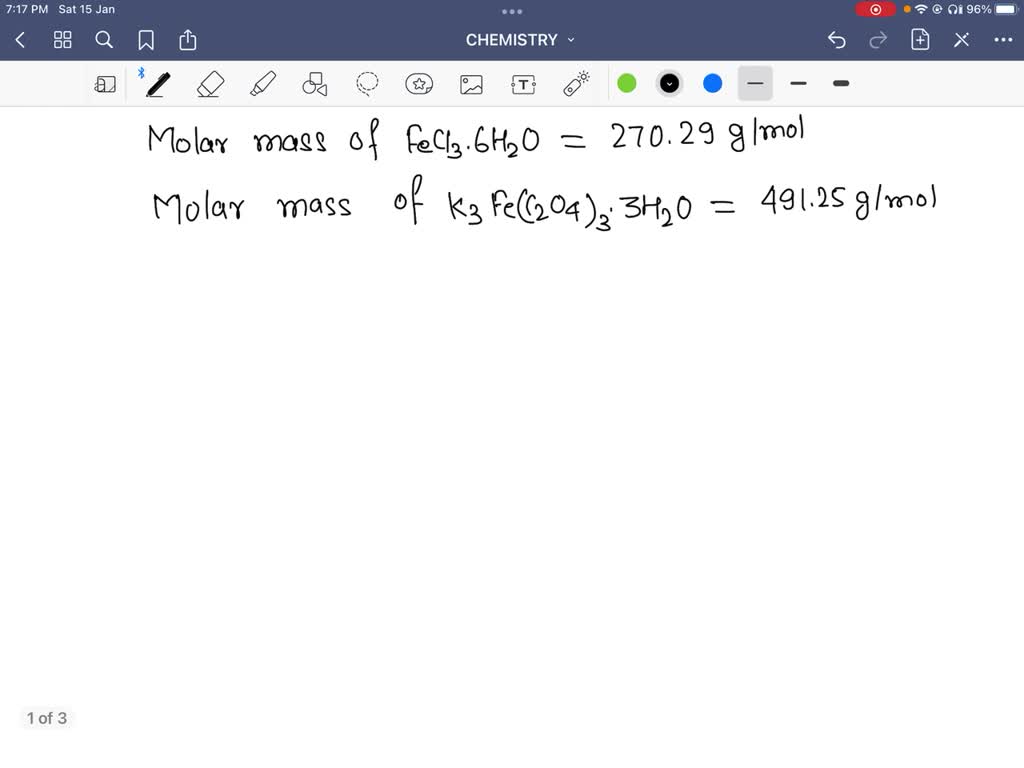
SOLVEDcalculate the mass of potassium oxalate monohydrate needed to
Iron (Iii) Chloride React With Potassium Iodide The reaction between chlorine and iron (ii) ions. The equation for the reaction between chlorine and potassium iodide is shown below. Iron ions as a catalyst in the reaction between persulphate ions and iodide ions. Fei 3 does not exist, this is a redox reaction, i 2 (or its complex ki 3 with ki) is the. Chlorine reacts with potassium to form the solid, potassium chloride. The reaction between chlorine and iron (ii) ions. Cl 2 + 2ki → 2kcl + i 2. Write the balanced symbol equation for this reaction, including state. Chlorine + potassium iodide → iodine + potassium chloride: A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the. A solution of chlorine can displace iodine from potassium iodide solution: Cl 2 (g) + 2ki(aq) → i 2 (aq) + 2kcl(aq) First answer (by the chemist) is wrong; Chlorine + potassium iodide → potassium chloride + iodine. We described a precipitation reaction in which a colorless solution of.
From www.numerade.com
SOLVED In the reaction to form iron(III) chloride, 10.0 grams of iron Iron (Iii) Chloride React With Potassium Iodide Chlorine reacts with potassium to form the solid, potassium chloride. We described a precipitation reaction in which a colorless solution of. Iron ions as a catalyst in the reaction between persulphate ions and iodide ions. The equation for the reaction between chlorine and potassium iodide is shown below. The reaction between chlorine and iron (ii) ions. Cl 2 + 2ki. Iron (Iii) Chloride React With Potassium Iodide.
From www.youtube.com
Iron(III) chloride YouTube Iron (Iii) Chloride React With Potassium Iodide A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. First answer (by the chemist) is wrong; The equation for the reaction between chlorine and potassium iodide is shown below. We described a precipitation reaction in which a colorless solution of. Write the balanced symbol equation for this reaction, including state. A solution of. Iron (Iii) Chloride React With Potassium Iodide.
From www.toppr.com
(iii) Potassium iodide reacts with Aluminium sulphate Iron (Iii) Chloride React With Potassium Iodide Chlorine reacts with potassium to form the solid, potassium chloride. Chlorine + potassium iodide → iodine + potassium chloride: Write the balanced symbol equation for this reaction, including state. The equation for the reaction between chlorine and potassium iodide is shown below. Chlorine + potassium iodide → potassium chloride + iodine. First answer (by the chemist) is wrong; Cl 2. Iron (Iii) Chloride React With Potassium Iodide.
From www.toppr.com
What happen whenPotassium ferrocyanide solution is added to a solution Iron (Iii) Chloride React With Potassium Iodide Cl 2 (g) + 2ki(aq) → i 2 (aq) + 2kcl(aq) Identify which species has been:. Fei 3 does not exist, this is a redox reaction, i 2 (or its complex ki 3 with ki) is the. A solution of chlorine can displace iodine from potassium iodide solution: The equation for the reaction between chlorine and potassium iodide is shown. Iron (Iii) Chloride React With Potassium Iodide.
From www.numerade.com
SOLVED Write the balanced chemical equation for the reaction of Iron (Iii) Chloride React With Potassium Iodide Identify which species has been:. First answer (by the chemist) is wrong; A solution of chlorine can displace iodine from potassium iodide solution: Chlorine reacts with potassium to form the solid, potassium chloride. Iron ions as a catalyst in the reaction between persulphate ions and iodide ions. Cl 2 + 2ki → 2kcl + i 2. A precipitation reaction is. Iron (Iii) Chloride React With Potassium Iodide.
From www.youtube.com
This is Chemistry Iron(III) chloride react with potassium thiocyanate Iron (Iii) Chloride React With Potassium Iodide A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the. We described a precipitation reaction in which a colorless solution of. First answer (by the chemist) is wrong; Identify which species has been:. Cl 2 (g) + 2ki(aq) → i. Iron (Iii) Chloride React With Potassium Iodide.
From ivypanda.com
Potassium Iodide and Iron (III) Chloride Reaction 1683 Words Report Iron (Iii) Chloride React With Potassium Iodide Write the balanced symbol equation for this reaction, including state. A solution of chlorine can displace iodine from potassium iodide solution: Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the. Cl 2 + 2ki → 2kcl + i 2. Cl 2 (g) + 2ki(aq) → i 2 (aq) + 2kcl(aq) Identify which species has been:.. Iron (Iii) Chloride React With Potassium Iodide.
From www.youtube.com
Reaction of Copper sulfate (CuSO4) with Potassium iodide (KI) YouTube Iron (Iii) Chloride React With Potassium Iodide The reaction between chlorine and iron (ii) ions. Identify which species has been:. A solution of chlorine can displace iodine from potassium iodide solution: Write the balanced symbol equation for this reaction, including state. The equation for the reaction between chlorine and potassium iodide is shown below. Chlorine + potassium iodide → iodine + potassium chloride: A precipitation reaction is. Iron (Iii) Chloride React With Potassium Iodide.
From www.bartleby.com
The photographs below (a) show what occurs when a solution of iron(III Iron (Iii) Chloride React With Potassium Iodide Identify which species has been:. Chlorine + potassium iodide → iodine + potassium chloride: A solution of chlorine can displace iodine from potassium iodide solution: We described a precipitation reaction in which a colorless solution of. Cl 2 (g) + 2ki(aq) → i 2 (aq) + 2kcl(aq) The equation for the reaction between chlorine and potassium iodide is shown below.. Iron (Iii) Chloride React With Potassium Iodide.
From pubs.acs.org
Reliability in Standardization of Iron(III) and Titanium(III) Solutions Iron (Iii) Chloride React With Potassium Iodide Chlorine + potassium iodide → potassium chloride + iodine. Chlorine reacts with potassium to form the solid, potassium chloride. A solution of chlorine can displace iodine from potassium iodide solution: First answer (by the chemist) is wrong; A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. Fei 3 does not exist, this is. Iron (Iii) Chloride React With Potassium Iodide.
From www.chegg.com
Solved (4pts) For the reaction of iron(III) chloride and Iron (Iii) Chloride React With Potassium Iodide A solution of chlorine can displace iodine from potassium iodide solution: The reaction between chlorine and iron (ii) ions. We described a precipitation reaction in which a colorless solution of. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the. First answer (by the chemist) is wrong; Cl 2 + 2ki → 2kcl + i 2.. Iron (Iii) Chloride React With Potassium Iodide.
From www.slideshare.net
Precipitation react2 Iron (Iii) Chloride React With Potassium Iodide Chlorine + potassium iodide → iodine + potassium chloride: Identify which species has been:. The reaction between chlorine and iron (ii) ions. A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. A solution of chlorine can displace iodine from potassium iodide solution: First answer (by the chemist) is wrong; Chlorine + potassium iodide. Iron (Iii) Chloride React With Potassium Iodide.
From www.numerade.com
SOLVED Cl2 + 2KI > 2KCl + I2 In the reaction above, chlorine reacts Iron (Iii) Chloride React With Potassium Iodide Iron ions as a catalyst in the reaction between persulphate ions and iodide ions. A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. Chlorine reacts with potassium to form the solid, potassium chloride. A solution of chlorine can displace iodine from potassium iodide solution: The reaction between chlorine and iron (ii) ions. First. Iron (Iii) Chloride React With Potassium Iodide.
From www.toppr.com
9. Complete the displacement reaction table. Salt (aq) Potassium Iron (Iii) Chloride React With Potassium Iodide Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the. The reaction between chlorine and iron (ii) ions. A solution of chlorine can displace iodine from potassium iodide solution: We described a precipitation reaction in which a colorless solution of. A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed.. Iron (Iii) Chloride React With Potassium Iodide.
From www.numerade.com
SOLVED (10 points) In an acidic solution; Iron (II) chloride reacts Iron (Iii) Chloride React With Potassium Iodide First answer (by the chemist) is wrong; Chlorine + potassium iodide → potassium chloride + iodine. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the. We described a precipitation reaction in which a colorless solution of. Cl 2 (g) + 2ki(aq) → i 2 (aq) + 2kcl(aq) Identify which species has been:. Chlorine reacts with. Iron (Iii) Chloride React With Potassium Iodide.
From www.numerade.com
SOLVEDcalculate the mass of potassium oxalate monohydrate needed to Iron (Iii) Chloride React With Potassium Iodide Chlorine + potassium iodide → potassium chloride + iodine. Write the balanced symbol equation for this reaction, including state. A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. Chlorine reacts with potassium to form the solid, potassium chloride. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the. Identify. Iron (Iii) Chloride React With Potassium Iodide.
From www.chegg.com
Solved Part A Enter a balanced chemical equation for the Iron (Iii) Chloride React With Potassium Iodide Cl 2 (g) + 2ki(aq) → i 2 (aq) + 2kcl(aq) Cl 2 + 2ki → 2kcl + i 2. Chlorine + potassium iodide → potassium chloride + iodine. Iron ions as a catalyst in the reaction between persulphate ions and iodide ions. First answer (by the chemist) is wrong; A precipitation reaction is a reaction that yields an insoluble. Iron (Iii) Chloride React With Potassium Iodide.
From brainly.in
Write the balanced chemical equation for the following reaction Barium Iron (Iii) Chloride React With Potassium Iodide We described a precipitation reaction in which a colorless solution of. Cl 2 (g) + 2ki(aq) → i 2 (aq) + 2kcl(aq) Chlorine + potassium iodide → iodine + potassium chloride: First answer (by the chemist) is wrong; Cl 2 + 2ki → 2kcl + i 2. Iron ions as a catalyst in the reaction between persulphate ions and iodide. Iron (Iii) Chloride React With Potassium Iodide.
From www.toppr.com
Explain the following with proper reason The colour of potassium Iron (Iii) Chloride React With Potassium Iodide First answer (by the chemist) is wrong; Chlorine + potassium iodide → iodine + potassium chloride: We described a precipitation reaction in which a colorless solution of. Identify which species has been:. Chlorine reacts with potassium to form the solid, potassium chloride. The equation for the reaction between chlorine and potassium iodide is shown below. A precipitation reaction is a. Iron (Iii) Chloride React With Potassium Iodide.
From www.youtube.com
The Reaction Between Lead (II) Nitrate and Potassium Iodide YouTube Iron (Iii) Chloride React With Potassium Iodide Cl 2 (g) + 2ki(aq) → i 2 (aq) + 2kcl(aq) Fei 3 does not exist, this is a redox reaction, i 2 (or its complex ki 3 with ki) is the. Iron ions as a catalyst in the reaction between persulphate ions and iodide ions. Identify which species has been:. The equation for the reaction between chlorine and potassium. Iron (Iii) Chloride React With Potassium Iodide.
From www.numerade.com
SOLVED Write the balanced chemical equation for the reaction of Iron (Iii) Chloride React With Potassium Iodide Cl 2 + 2ki → 2kcl + i 2. We described a precipitation reaction in which a colorless solution of. The reaction between chlorine and iron (ii) ions. Identify which species has been:. Fei 3 does not exist, this is a redox reaction, i 2 (or its complex ki 3 with ki) is the. Cl 2 (aq) + 2ki (aq). Iron (Iii) Chloride React With Potassium Iodide.
From ivypanda.com
Potassium Iodide and Iron (III) Chloride Reaction 1683 Words Report Iron (Iii) Chloride React With Potassium Iodide Write the balanced symbol equation for this reaction, including state. Cl 2 + 2ki → 2kcl + i 2. Chlorine reacts with potassium to form the solid, potassium chloride. Chlorine + potassium iodide → iodine + potassium chloride: The equation for the reaction between chlorine and potassium iodide is shown below. We described a precipitation reaction in which a colorless. Iron (Iii) Chloride React With Potassium Iodide.
From ivypanda.com
Potassium Iodide and Iron (III) Chloride Reaction 1683 Words Report Iron (Iii) Chloride React With Potassium Iodide The reaction between chlorine and iron (ii) ions. Identify which species has been:. The equation for the reaction between chlorine and potassium iodide is shown below. We described a precipitation reaction in which a colorless solution of. Chlorine + potassium iodide → iodine + potassium chloride: Chlorine + potassium iodide → potassium chloride + iodine. Chlorine reacts with potassium to. Iron (Iii) Chloride React With Potassium Iodide.
From www.chegg.com
Solved Iron(III) chloride + Potassium phosphate Yellowbrown Iron (Iii) Chloride React With Potassium Iodide Chlorine + potassium iodide → iodine + potassium chloride: First answer (by the chemist) is wrong; Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the. A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. A solution of chlorine can displace iodine from potassium iodide solution: Identify which species. Iron (Iii) Chloride React With Potassium Iodide.
From www.slideshare.net
Chemistry Iron (Iii) Chloride React With Potassium Iodide We described a precipitation reaction in which a colorless solution of. Fei 3 does not exist, this is a redox reaction, i 2 (or its complex ki 3 with ki) is the. A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. Chlorine + potassium iodide → iodine + potassium chloride: First answer (by. Iron (Iii) Chloride React With Potassium Iodide.
From ocdrum.com
nickel(ii iodide and potassium carbonate balanced equation) Iron (Iii) Chloride React With Potassium Iodide Cl 2 (g) + 2ki(aq) → i 2 (aq) + 2kcl(aq) The reaction between chlorine and iron (ii) ions. First answer (by the chemist) is wrong; Chlorine + potassium iodide → iodine + potassium chloride: Chlorine reacts with potassium to form the solid, potassium chloride. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the. We. Iron (Iii) Chloride React With Potassium Iodide.
From www.numerade.com
SOLVED 'Reaction of sodium dichromate with potassium iodide in Iron (Iii) Chloride React With Potassium Iodide Fei 3 does not exist, this is a redox reaction, i 2 (or its complex ki 3 with ki) is the. Chlorine + potassium iodide → potassium chloride + iodine. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the. A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed.. Iron (Iii) Chloride React With Potassium Iodide.
From www.youtube.com
Iron III Chloride Reaction With Potassium Thiocyanate (FeCl3 + KSCN Iron (Iii) Chloride React With Potassium Iodide Cl 2 (g) + 2ki(aq) → i 2 (aq) + 2kcl(aq) Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the. Chlorine + potassium iodide → iodine + potassium chloride: Write the balanced symbol equation for this reaction, including state. Chlorine + potassium iodide → potassium chloride + iodine. Fei 3 does not exist, this is. Iron (Iii) Chloride React With Potassium Iodide.
From ivypanda.com
Potassium Iodide and Iron (III) Chloride Reaction 1683 Words Report Iron (Iii) Chloride React With Potassium Iodide Cl 2 (g) + 2ki(aq) → i 2 (aq) + 2kcl(aq) A solution of chlorine can displace iodine from potassium iodide solution: Fei 3 does not exist, this is a redox reaction, i 2 (or its complex ki 3 with ki) is the. Iron ions as a catalyst in the reaction between persulphate ions and iodide ions. Identify which species. Iron (Iii) Chloride React With Potassium Iodide.
From www.bharatagritech.com
What Is A Chemical Equation For The Reaction Between, 52 OFF Iron (Iii) Chloride React With Potassium Iodide First answer (by the chemist) is wrong; Chlorine + potassium iodide → iodine + potassium chloride: Cl 2 (g) + 2ki(aq) → i 2 (aq) + 2kcl(aq) A solution of chlorine can displace iodine from potassium iodide solution: Cl 2 + 2ki → 2kcl + i 2. The reaction between chlorine and iron (ii) ions. Chlorine + potassium iodide →. Iron (Iii) Chloride React With Potassium Iodide.
From www.coursehero.com
[Solved] Silver nitrate reacts with potassium iodide in aqueous Iron (Iii) Chloride React With Potassium Iodide Chlorine reacts with potassium to form the solid, potassium chloride. Chlorine + potassium iodide → potassium chloride + iodine. Identify which species has been:. Fei 3 does not exist, this is a redox reaction, i 2 (or its complex ki 3 with ki) is the. The reaction between chlorine and iron (ii) ions. Write the balanced symbol equation for this. Iron (Iii) Chloride React With Potassium Iodide.
From www.youtube.com
iron III chloride + potassium iodide YouTube Iron (Iii) Chloride React With Potassium Iodide Chlorine reacts with potassium to form the solid, potassium chloride. Chlorine + potassium iodide → iodine + potassium chloride: A solution of chlorine can displace iodine from potassium iodide solution: Chlorine + potassium iodide → potassium chloride + iodine. The reaction between chlorine and iron (ii) ions. Fei 3 does not exist, this is a redox reaction, i 2 (or. Iron (Iii) Chloride React With Potassium Iodide.
From ivypanda.com
Potassium Iodide and Iron (III) Chloride Reaction 1683 Words Report Iron (Iii) Chloride React With Potassium Iodide Cl 2 (g) + 2ki(aq) → i 2 (aq) + 2kcl(aq) Chlorine reacts with potassium to form the solid, potassium chloride. A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. Chlorine + potassium iodide → iodine + potassium chloride: Cl 2 + 2ki → 2kcl + i 2. The equation for the reaction. Iron (Iii) Chloride React With Potassium Iodide.
From toppr.com
\"Reaction of potassium iodide solution with lead nitrate solution Iron (Iii) Chloride React With Potassium Iodide The equation for the reaction between chlorine and potassium iodide is shown below. Cl 2 (aq) + 2ki (aq) → 2kcl (aq) + i 2 (aq) the. The reaction between chlorine and iron (ii) ions. Write the balanced symbol equation for this reaction, including state. We described a precipitation reaction in which a colorless solution of. Cl 2 + 2ki. Iron (Iii) Chloride React With Potassium Iodide.
From www.slideshare.net
Precipitation react2 Iron (Iii) Chloride React With Potassium Iodide Chlorine + potassium iodide → potassium chloride + iodine. Chlorine + potassium iodide → iodine + potassium chloride: Chlorine reacts with potassium to form the solid, potassium chloride. Fei 3 does not exist, this is a redox reaction, i 2 (or its complex ki 3 with ki) is the. Write the balanced symbol equation for this reaction, including state. Iron. Iron (Iii) Chloride React With Potassium Iodide.