Sodium Or Chlorine Ionization Energy . For example, sodium requires only 496 kj/mol or 5.14. Ionization energy increases across a row on the periodic maximum for the noble gases which have closed shells. Ionization energy is a measure of the energy needed to pull a particular electron away from the attraction of the nucleus. In a chemical reaction, understanding ionization energy is important in order to understand the behavior of whether various atoms make. In physics and chemistry, ionization energy (ie) is the minimum energy required to remove the most loosely bound electron of an isolated gaseous. These tables list values of molar ionization energies, measured in kj⋅mol −1. This is the energy per mole necessary to remove electrons. 1st ie = 495.8 kj/mol cl: First ionization energy, second ionization energy as well as. Ionization energies of the elements in the third row of the periodic table exhibit the same pattern as those of \(li\) and \(be\) (table \(\pageindex{2}\)): A high value of ionization energy shows a high attraction between the. 120 rows ionization energy chart of all the elements is given below.
from www.pikpng.com
1st ie = 495.8 kj/mol cl: In physics and chemistry, ionization energy (ie) is the minimum energy required to remove the most loosely bound electron of an isolated gaseous. 120 rows ionization energy chart of all the elements is given below. Ionization energy is a measure of the energy needed to pull a particular electron away from the attraction of the nucleus. For example, sodium requires only 496 kj/mol or 5.14. These tables list values of molar ionization energies, measured in kj⋅mol −1. In a chemical reaction, understanding ionization energy is important in order to understand the behavior of whether various atoms make. First ionization energy, second ionization energy as well as. A high value of ionization energy shows a high attraction between the. Ionization energy increases across a row on the periodic maximum for the noble gases which have closed shells.
Download Png Royalty Free Drawing Atoms Sodium Covalent Bonding Of
Sodium Or Chlorine Ionization Energy 1st ie = 495.8 kj/mol cl: First ionization energy, second ionization energy as well as. Ionization energy is a measure of the energy needed to pull a particular electron away from the attraction of the nucleus. A high value of ionization energy shows a high attraction between the. For example, sodium requires only 496 kj/mol or 5.14. 120 rows ionization energy chart of all the elements is given below. Ionization energy increases across a row on the periodic maximum for the noble gases which have closed shells. This is the energy per mole necessary to remove electrons. 1st ie = 495.8 kj/mol cl: In physics and chemistry, ionization energy (ie) is the minimum energy required to remove the most loosely bound electron of an isolated gaseous. These tables list values of molar ionization energies, measured in kj⋅mol −1. Ionization energies of the elements in the third row of the periodic table exhibit the same pattern as those of \(li\) and \(be\) (table \(\pageindex{2}\)): In a chemical reaction, understanding ionization energy is important in order to understand the behavior of whether various atoms make.
From mmerevise.co.uk
Ionisation Energies MME Sodium Or Chlorine Ionization Energy This is the energy per mole necessary to remove electrons. Ionization energy increases across a row on the periodic maximum for the noble gases which have closed shells. Ionization energy is a measure of the energy needed to pull a particular electron away from the attraction of the nucleus. 1st ie = 495.8 kj/mol cl: First ionization energy, second ionization. Sodium Or Chlorine Ionization Energy.
From www.alamy.com
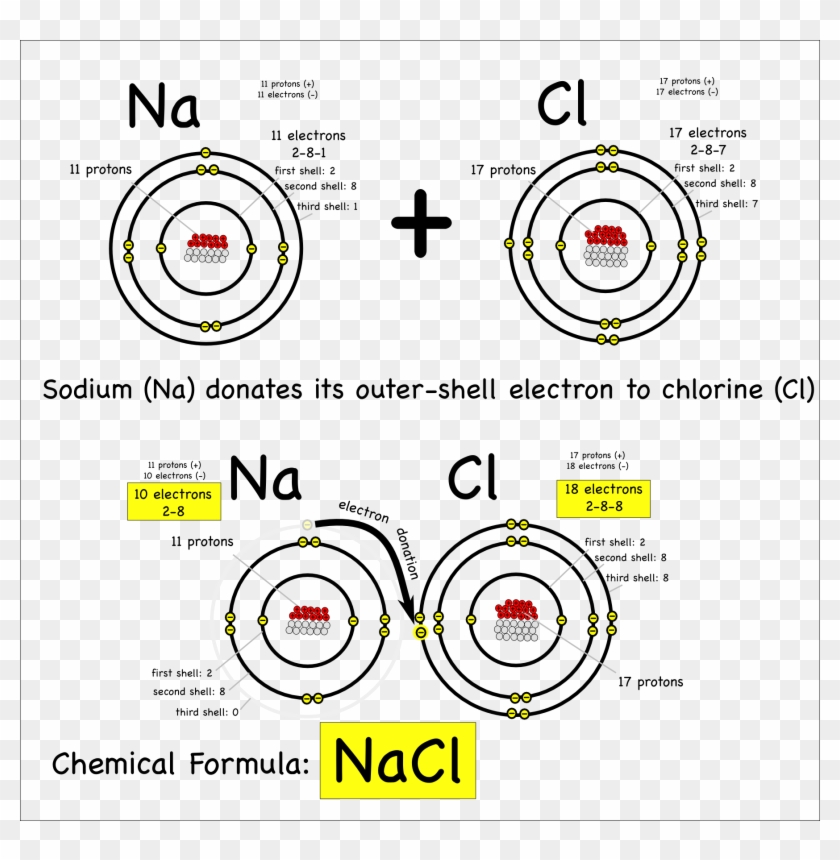
Diagram to show ionic bonding in sodium chloride Stock Vector Image Sodium Or Chlorine Ionization Energy 120 rows ionization energy chart of all the elements is given below. These tables list values of molar ionization energies, measured in kj⋅mol −1. In a chemical reaction, understanding ionization energy is important in order to understand the behavior of whether various atoms make. Ionization energies of the elements in the third row of the periodic table exhibit the same. Sodium Or Chlorine Ionization Energy.
From www.shutterstock.com
Chlorine Chemical Element First Ionization Energy เวกเตอร์สต็อก (ปลอด Sodium Or Chlorine Ionization Energy In a chemical reaction, understanding ionization energy is important in order to understand the behavior of whether various atoms make. Ionization energies of the elements in the third row of the periodic table exhibit the same pattern as those of \(li\) and \(be\) (table \(\pageindex{2}\)): This is the energy per mole necessary to remove electrons. For example, sodium requires only. Sodium Or Chlorine Ionization Energy.
From byjus.com
What is the magnitude of force between a singly charged sodium ion and Sodium Or Chlorine Ionization Energy In physics and chemistry, ionization energy (ie) is the minimum energy required to remove the most loosely bound electron of an isolated gaseous. First ionization energy, second ionization energy as well as. In a chemical reaction, understanding ionization energy is important in order to understand the behavior of whether various atoms make. Ionization energy increases across a row on the. Sodium Or Chlorine Ionization Energy.
From www.youtube.com
S1.3.7 Successive ionisation energies (HL) YouTube Sodium Or Chlorine Ionization Energy Ionization energies of the elements in the third row of the periodic table exhibit the same pattern as those of \(li\) and \(be\) (table \(\pageindex{2}\)): Ionization energy increases across a row on the periodic maximum for the noble gases which have closed shells. In physics and chemistry, ionization energy (ie) is the minimum energy required to remove the most loosely. Sodium Or Chlorine Ionization Energy.
From www.sciencenewsforstudents.org
Explainer Ions and radicals in our world Science News for Students Sodium Or Chlorine Ionization Energy In physics and chemistry, ionization energy (ie) is the minimum energy required to remove the most loosely bound electron of an isolated gaseous. In a chemical reaction, understanding ionization energy is important in order to understand the behavior of whether various atoms make. For example, sodium requires only 496 kj/mol or 5.14. Ionization energies of the elements in the third. Sodium Or Chlorine Ionization Energy.
From askfilo.com
The first ionisation energy of chlorine is greater than that of sulfur.W.. Sodium Or Chlorine Ionization Energy This is the energy per mole necessary to remove electrons. First ionization energy, second ionization energy as well as. Ionization energy increases across a row on the periodic maximum for the noble gases which have closed shells. A high value of ionization energy shows a high attraction between the. 120 rows ionization energy chart of all the elements is given. Sodium Or Chlorine Ionization Energy.
From www.differencebetween.com
Difference Between First and Second Ionization Energy (I1E vs I2E Sodium Or Chlorine Ionization Energy Ionization energy increases across a row on the periodic maximum for the noble gases which have closed shells. For example, sodium requires only 496 kj/mol or 5.14. 1st ie = 495.8 kj/mol cl: This is the energy per mole necessary to remove electrons. In a chemical reaction, understanding ionization energy is important in order to understand the behavior of whether. Sodium Or Chlorine Ionization Energy.
From chem.libretexts.org
4.3 The Reaction of Sodium with Chlorine Chemistry LibreTexts Sodium Or Chlorine Ionization Energy Ionization energy increases across a row on the periodic maximum for the noble gases which have closed shells. First ionization energy, second ionization energy as well as. 1st ie = 495.8 kj/mol cl: For example, sodium requires only 496 kj/mol or 5.14. In physics and chemistry, ionization energy (ie) is the minimum energy required to remove the most loosely bound. Sodium Or Chlorine Ionization Energy.
From www.tessshebaylo.com
Write An Equation With State Symbols To Represent The Second Ionisation Sodium Or Chlorine Ionization Energy In physics and chemistry, ionization energy (ie) is the minimum energy required to remove the most loosely bound electron of an isolated gaseous. In a chemical reaction, understanding ionization energy is important in order to understand the behavior of whether various atoms make. Ionization energy is a measure of the energy needed to pull a particular electron away from the. Sodium Or Chlorine Ionization Energy.
From www.pikpng.com
Download Png Royalty Free Drawing Atoms Sodium Covalent Bonding Of Sodium Or Chlorine Ionization Energy Ionization energy is a measure of the energy needed to pull a particular electron away from the attraction of the nucleus. This is the energy per mole necessary to remove electrons. 1st ie = 495.8 kj/mol cl: A high value of ionization energy shows a high attraction between the. In a chemical reaction, understanding ionization energy is important in order. Sodium Or Chlorine Ionization Energy.
From www.dreamstime.com
Ionic Bonding in a Solid Sodium Chloride Crystal Stock Vector Sodium Or Chlorine Ionization Energy 1st ie = 495.8 kj/mol cl: Ionization energy increases across a row on the periodic maximum for the noble gases which have closed shells. For example, sodium requires only 496 kj/mol or 5.14. Ionization energies of the elements in the third row of the periodic table exhibit the same pattern as those of \(li\) and \(be\) (table \(\pageindex{2}\)): These tables. Sodium Or Chlorine Ionization Energy.
From www.numerade.com
SOLVED Rank the following elements according to their ionization Sodium Or Chlorine Ionization Energy Ionization energy is a measure of the energy needed to pull a particular electron away from the attraction of the nucleus. This is the energy per mole necessary to remove electrons. In a chemical reaction, understanding ionization energy is important in order to understand the behavior of whether various atoms make. First ionization energy, second ionization energy as well as.. Sodium Or Chlorine Ionization Energy.
From www.periodic-table.org
Chlorine Ionization Energy Sodium Or Chlorine Ionization Energy In physics and chemistry, ionization energy (ie) is the minimum energy required to remove the most loosely bound electron of an isolated gaseous. For example, sodium requires only 496 kj/mol or 5.14. 120 rows ionization energy chart of all the elements is given below. A high value of ionization energy shows a high attraction between the. 1st ie = 495.8. Sodium Or Chlorine Ionization Energy.
From www.nuclear-power.com
Chlorine Electron Affinity Electronegativity Ionization Energy of Sodium Or Chlorine Ionization Energy These tables list values of molar ionization energies, measured in kj⋅mol −1. This is the energy per mole necessary to remove electrons. 1st ie = 495.8 kj/mol cl: For example, sodium requires only 496 kj/mol or 5.14. A high value of ionization energy shows a high attraction between the. First ionization energy, second ionization energy as well as. Ionization energies. Sodium Or Chlorine Ionization Energy.
From slideplayer.com
Trends in Periodic Table ppt download Sodium Or Chlorine Ionization Energy First ionization energy, second ionization energy as well as. In a chemical reaction, understanding ionization energy is important in order to understand the behavior of whether various atoms make. In physics and chemistry, ionization energy (ie) is the minimum energy required to remove the most loosely bound electron of an isolated gaseous. Ionization energies of the elements in the third. Sodium Or Chlorine Ionization Energy.
From chemwiki.ucdavis.edu
Periodic Properties of the Elements Chemwiki Sodium Or Chlorine Ionization Energy This is the energy per mole necessary to remove electrons. Ionization energy is a measure of the energy needed to pull a particular electron away from the attraction of the nucleus. First ionization energy, second ionization energy as well as. In a chemical reaction, understanding ionization energy is important in order to understand the behavior of whether various atoms make.. Sodium Or Chlorine Ionization Energy.
From www.chemistrylearner.com
Ionization Energy Definition, Chart & Periodic Table Trend Sodium Or Chlorine Ionization Energy In a chemical reaction, understanding ionization energy is important in order to understand the behavior of whether various atoms make. These tables list values of molar ionization energies, measured in kj⋅mol −1. First ionization energy, second ionization energy as well as. A high value of ionization energy shows a high attraction between the. Ionization energy is a measure of the. Sodium Or Chlorine Ionization Energy.
From www.slideserve.com
PPT Lattice Energy & the BornHaber Cycle PowerPoint Presentation Sodium Or Chlorine Ionization Energy First ionization energy, second ionization energy as well as. In a chemical reaction, understanding ionization energy is important in order to understand the behavior of whether various atoms make. 1st ie = 495.8 kj/mol cl: This is the energy per mole necessary to remove electrons. For example, sodium requires only 496 kj/mol or 5.14. 120 rows ionization energy chart of. Sodium Or Chlorine Ionization Energy.
From www.numerade.com
SOLVED 8. Which of the following has the highest ionization energy? â Sodium Or Chlorine Ionization Energy Ionization energy is a measure of the energy needed to pull a particular electron away from the attraction of the nucleus. A high value of ionization energy shows a high attraction between the. In a chemical reaction, understanding ionization energy is important in order to understand the behavior of whether various atoms make. These tables list values of molar ionization. Sodium Or Chlorine Ionization Energy.
From www.numerade.com
The value of the second ionization energy of calcium is 1150 KJ/mol Sodium Or Chlorine Ionization Energy This is the energy per mole necessary to remove electrons. First ionization energy, second ionization energy as well as. For example, sodium requires only 496 kj/mol or 5.14. Ionization energies of the elements in the third row of the periodic table exhibit the same pattern as those of \(li\) and \(be\) (table \(\pageindex{2}\)): Ionization energy increases across a row on. Sodium Or Chlorine Ionization Energy.
From www.animalia-life.club
Second Ionization Energy Periodic Table Sodium Or Chlorine Ionization Energy A high value of ionization energy shows a high attraction between the. 1st ie = 495.8 kj/mol cl: First ionization energy, second ionization energy as well as. Ionization energy increases across a row on the periodic maximum for the noble gases which have closed shells. In a chemical reaction, understanding ionization energy is important in order to understand the behavior. Sodium Or Chlorine Ionization Energy.
From neetlab.com
Ionization Enthalpy NEET Lab Sodium Or Chlorine Ionization Energy 120 rows ionization energy chart of all the elements is given below. Ionization energies of the elements in the third row of the periodic table exhibit the same pattern as those of \(li\) and \(be\) (table \(\pageindex{2}\)): Ionization energy increases across a row on the periodic maximum for the noble gases which have closed shells. These tables list values of. Sodium Or Chlorine Ionization Energy.
From chemistnotes.com
Periodic table Archives Chemistry Notes Sodium Or Chlorine Ionization Energy For example, sodium requires only 496 kj/mol or 5.14. In a chemical reaction, understanding ionization energy is important in order to understand the behavior of whether various atoms make. Ionization energy increases across a row on the periodic maximum for the noble gases which have closed shells. Ionization energies of the elements in the third row of the periodic table. Sodium Or Chlorine Ionization Energy.
From www.numerade.com
SOLVED Homework Question 3.49 Homework Uranswvered Rank the following Sodium Or Chlorine Ionization Energy 120 rows ionization energy chart of all the elements is given below. In a chemical reaction, understanding ionization energy is important in order to understand the behavior of whether various atoms make. For example, sodium requires only 496 kj/mol or 5.14. First ionization energy, second ionization energy as well as. 1st ie = 495.8 kj/mol cl: This is the energy. Sodium Or Chlorine Ionization Energy.
From www.linstitute.net
AQA A Level Chemistry复习笔记1.1.7 Ionisation Energy Trends & Evidence翰林国际教育 Sodium Or Chlorine Ionization Energy For example, sodium requires only 496 kj/mol or 5.14. This is the energy per mole necessary to remove electrons. 1st ie = 495.8 kj/mol cl: 120 rows ionization energy chart of all the elements is given below. In physics and chemistry, ionization energy (ie) is the minimum energy required to remove the most loosely bound electron of an isolated gaseous.. Sodium Or Chlorine Ionization Energy.
From slideplayer.com
Introduction to Periodic Table ppt download Sodium Or Chlorine Ionization Energy In physics and chemistry, ionization energy (ie) is the minimum energy required to remove the most loosely bound electron of an isolated gaseous. These tables list values of molar ionization energies, measured in kj⋅mol −1. 120 rows ionization energy chart of all the elements is given below. Ionization energy is a measure of the energy needed to pull a particular. Sodium Or Chlorine Ionization Energy.
From www.numerade.com
SOLVED QUESTION 5 (Start on a new page. The first ionisation energy Sodium Or Chlorine Ionization Energy These tables list values of molar ionization energies, measured in kj⋅mol −1. 1st ie = 495.8 kj/mol cl: This is the energy per mole necessary to remove electrons. 120 rows ionization energy chart of all the elements is given below. Ionization energy is a measure of the energy needed to pull a particular electron away from the attraction of the. Sodium Or Chlorine Ionization Energy.
From www.ck12.org
Periodic Trends in Ionization Energy CK12 Foundation Sodium Or Chlorine Ionization Energy This is the energy per mole necessary to remove electrons. Ionization energy increases across a row on the periodic maximum for the noble gases which have closed shells. For example, sodium requires only 496 kj/mol or 5.14. First ionization energy, second ionization energy as well as. Ionization energy is a measure of the energy needed to pull a particular electron. Sodium Or Chlorine Ionization Energy.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Sodium Or Chlorine Ionization Energy First ionization energy, second ionization energy as well as. 120 rows ionization energy chart of all the elements is given below. These tables list values of molar ionization energies, measured in kj⋅mol −1. A high value of ionization energy shows a high attraction between the. 1st ie = 495.8 kj/mol cl: For example, sodium requires only 496 kj/mol or 5.14.. Sodium Or Chlorine Ionization Energy.
From general.chemistrysteps.com
Electron Affinity Chemistry Steps Sodium Or Chlorine Ionization Energy This is the energy per mole necessary to remove electrons. Ionization energies of the elements in the third row of the periodic table exhibit the same pattern as those of \(li\) and \(be\) (table \(\pageindex{2}\)): These tables list values of molar ionization energies, measured in kj⋅mol −1. A high value of ionization energy shows a high attraction between the. For. Sodium Or Chlorine Ionization Energy.
From www.alamy.com
Sodium Chloride ionic bond formation. NaCl structure. Sodium and Sodium Or Chlorine Ionization Energy A high value of ionization energy shows a high attraction between the. This is the energy per mole necessary to remove electrons. Ionization energy is a measure of the energy needed to pull a particular electron away from the attraction of the nucleus. In physics and chemistry, ionization energy (ie) is the minimum energy required to remove the most loosely. Sodium Or Chlorine Ionization Energy.
From www.pinterest.com
Ionization Energy the amount of energy required to remove an electron Sodium Or Chlorine Ionization Energy Ionization energy is a measure of the energy needed to pull a particular electron away from the attraction of the nucleus. 1st ie = 495.8 kj/mol cl: In physics and chemistry, ionization energy (ie) is the minimum energy required to remove the most loosely bound electron of an isolated gaseous. For example, sodium requires only 496 kj/mol or 5.14. These. Sodium Or Chlorine Ionization Energy.
From winter.group.shef.ac.uk
Elements Periodic Table » Chlorine » properties of free atoms Sodium Or Chlorine Ionization Energy 1st ie = 495.8 kj/mol cl: 120 rows ionization energy chart of all the elements is given below. In a chemical reaction, understanding ionization energy is important in order to understand the behavior of whether various atoms make. Ionization energies of the elements in the third row of the periodic table exhibit the same pattern as those of \(li\) and. Sodium Or Chlorine Ionization Energy.
From www.geeksforgeeks.org
Ionization Energy Definition, Formulas, and Solved Examples Sodium Or Chlorine Ionization Energy 120 rows ionization energy chart of all the elements is given below. These tables list values of molar ionization energies, measured in kj⋅mol −1. For example, sodium requires only 496 kj/mol or 5.14. First ionization energy, second ionization energy as well as. In a chemical reaction, understanding ionization energy is important in order to understand the behavior of whether various. Sodium Or Chlorine Ionization Energy.