Calorimeter Constant For Water . liquid water has one of the highest specific heats known. pour in a known quantity of hot water (m 1) into a calorimeter containing a known quantity of cold water (m 2) and record the equilibrium. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. the calorimetry calculator can help you solve complex calorimetry problems. the calorimeter constant for the calorimeter is 36.0 j/°c and you can obtain the specific heat capacities for gold and water from table 5.2.1. after mixing 100.0 g of water at 58.5 °c with 100.0 g of water, already in the calorimeter, at 22.8 °c, the final temperature of the. It can analyze the heat exchange between up to 3 objects. 5.6.10 for \(t_f\), but there are two entities absorbing the heat \[ q_c +q_h=0 \\ (m_cc_c \delta t _c + c_{cal} \delta t _c) + m_hc_h \delta t_h=0 \] the calorimeter constant is the net heat capacity of the bomb, the thermostat water, the bucket, and the.
from www.chegg.com
pour in a known quantity of hot water (m 1) into a calorimeter containing a known quantity of cold water (m 2) and record the equilibrium. It can analyze the heat exchange between up to 3 objects. after mixing 100.0 g of water at 58.5 °c with 100.0 g of water, already in the calorimeter, at 22.8 °c, the final temperature of the. 5.6.10 for \(t_f\), but there are two entities absorbing the heat \[ q_c +q_h=0 \\ (m_cc_c \delta t _c + c_{cal} \delta t _c) + m_hc_h \delta t_h=0 \] the calorimeter constant is the net heat capacity of the bomb, the thermostat water, the bucket, and the. the calorimetry calculator can help you solve complex calorimetry problems. liquid water has one of the highest specific heats known. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. the calorimeter constant for the calorimeter is 36.0 j/°c and you can obtain the specific heat capacities for gold and water from table 5.2.1.
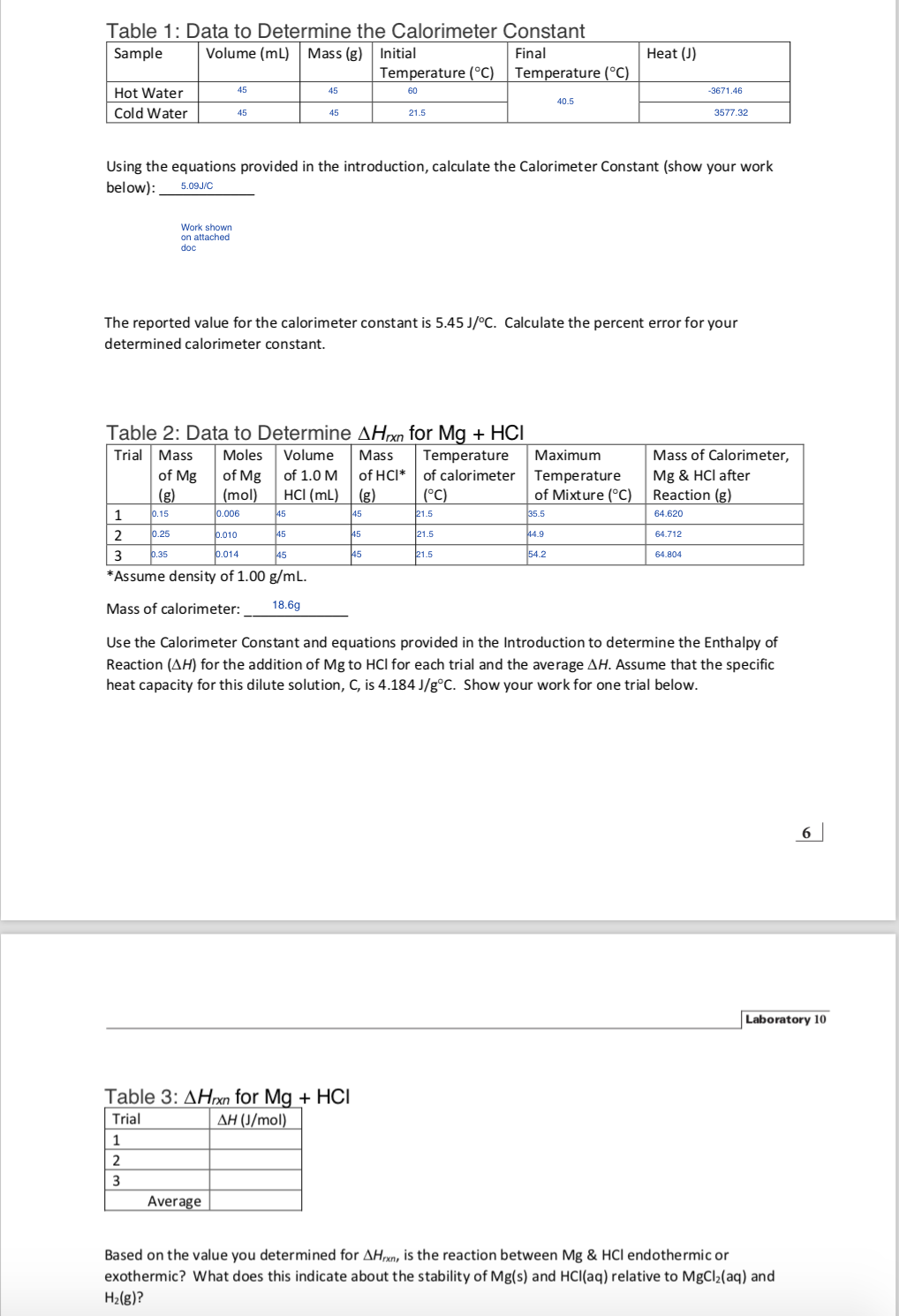
Solved Use the Calorimeter Constant and equations provided
Calorimeter Constant For Water after mixing 100.0 g of water at 58.5 °c with 100.0 g of water, already in the calorimeter, at 22.8 °c, the final temperature of the. liquid water has one of the highest specific heats known. the calorimeter constant for the calorimeter is 36.0 j/°c and you can obtain the specific heat capacities for gold and water from table 5.2.1. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. the calorimeter constant is the net heat capacity of the bomb, the thermostat water, the bucket, and the. It can analyze the heat exchange between up to 3 objects. after mixing 100.0 g of water at 58.5 °c with 100.0 g of water, already in the calorimeter, at 22.8 °c, the final temperature of the. pour in a known quantity of hot water (m 1) into a calorimeter containing a known quantity of cold water (m 2) and record the equilibrium. 5.6.10 for \(t_f\), but there are two entities absorbing the heat \[ q_c +q_h=0 \\ (m_cc_c \delta t _c + c_{cal} \delta t _c) + m_hc_h \delta t_h=0 \] the calorimetry calculator can help you solve complex calorimetry problems.
From saylordotorg.github.io
Calorimetry Calorimeter Constant For Water the calorimeter constant is the net heat capacity of the bomb, the thermostat water, the bucket, and the. after mixing 100.0 g of water at 58.5 °c with 100.0 g of water, already in the calorimeter, at 22.8 °c, the final temperature of the. the calorimetry calculator can help you solve complex calorimetry problems. the calorimeter. Calorimeter Constant For Water.
From exydhmsea.blob.core.windows.net
Calorimeter Enthalpy Of Vaporization at John Martinez blog Calorimeter Constant For Water after mixing 100.0 g of water at 58.5 °c with 100.0 g of water, already in the calorimeter, at 22.8 °c, the final temperature of the. pour in a known quantity of hot water (m 1) into a calorimeter containing a known quantity of cold water (m 2) and record the equilibrium. It can analyze the heat exchange. Calorimeter Constant For Water.
From hxemxcuhb.blob.core.windows.net
Equation In Calorimeter at John Hanna blog Calorimeter Constant For Water 5.6.10 for \(t_f\), but there are two entities absorbing the heat \[ q_c +q_h=0 \\ (m_cc_c \delta t _c + c_{cal} \delta t _c) + m_hc_h \delta t_h=0 \] a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. the calorimeter constant for the calorimeter is 36.0 j/°c and. Calorimeter Constant For Water.
From www.chegg.com
Solved Use the Calorimeter Constant and equations provided Calorimeter Constant For Water after mixing 100.0 g of water at 58.5 °c with 100.0 g of water, already in the calorimeter, at 22.8 °c, the final temperature of the. 5.6.10 for \(t_f\), but there are two entities absorbing the heat \[ q_c +q_h=0 \\ (m_cc_c \delta t _c + c_{cal} \delta t _c) + m_hc_h \delta t_h=0 \] pour in a. Calorimeter Constant For Water.
From www.chegg.com
Chemistry Archive October 31, 2017 Calorimeter Constant For Water after mixing 100.0 g of water at 58.5 °c with 100.0 g of water, already in the calorimeter, at 22.8 °c, the final temperature of the. the calorimeter constant is the net heat capacity of the bomb, the thermostat water, the bucket, and the. 5.6.10 for \(t_f\), but there are two entities absorbing the heat \[ q_c +q_h=0. Calorimeter Constant For Water.
From www.youtube.com
How to find calorimeter constant YouTube Calorimeter Constant For Water liquid water has one of the highest specific heats known. the calorimeter constant for the calorimeter is 36.0 j/°c and you can obtain the specific heat capacities for gold and water from table 5.2.1. after mixing 100.0 g of water at 58.5 °c with 100.0 g of water, already in the calorimeter, at 22.8 °c, the final. Calorimeter Constant For Water.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3850751 Calorimeter Constant For Water 5.6.10 for \(t_f\), but there are two entities absorbing the heat \[ q_c +q_h=0 \\ (m_cc_c \delta t _c + c_{cal} \delta t _c) + m_hc_h \delta t_h=0 \] It can analyze the heat exchange between up to 3 objects. pour in a known quantity of hot water (m 1) into a calorimeter containing a known quantity of cold. Calorimeter Constant For Water.
From www.numerade.com
SOLVEDAnalyeis Calculate the calorimeter constant (C) for your Calorimeter Constant For Water It can analyze the heat exchange between up to 3 objects. liquid water has one of the highest specific heats known. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. the calorimetry calculator can help you solve complex calorimetry problems. the calorimeter constant for the calorimeter. Calorimeter Constant For Water.
From www.numerade.com
SOLVED Lab Notebook Calorimeter Constant Determination Calculation Calorimeter Constant For Water the calorimeter constant for the calorimeter is 36.0 j/°c and you can obtain the specific heat capacities for gold and water from table 5.2.1. the calorimeter constant is the net heat capacity of the bomb, the thermostat water, the bucket, and the. a calorimeter is a device used to measure the amount of heat involved in a. Calorimeter Constant For Water.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimeter Constant For Water It can analyze the heat exchange between up to 3 objects. liquid water has one of the highest specific heats known. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. after mixing 100.0 g of water at 58.5 °c with 100.0 g of water, already in the. Calorimeter Constant For Water.
From www.slideserve.com
PPT Zumdahl Chapter 6 PowerPoint Presentation, free download ID4269919 Calorimeter Constant For Water a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. liquid water has one of the highest specific heats known. the calorimeter constant for the calorimeter is 36.0 j/°c and you can obtain the specific heat capacities for gold and water from table 5.2.1. It can analyze the. Calorimeter Constant For Water.
From hxentbteo.blob.core.windows.net
Calorimeter Experiment Calculations at Jerry Obrien blog Calorimeter Constant For Water pour in a known quantity of hot water (m 1) into a calorimeter containing a known quantity of cold water (m 2) and record the equilibrium. liquid water has one of the highest specific heats known. 5.6.10 for \(t_f\), but there are two entities absorbing the heat \[ q_c +q_h=0 \\ (m_cc_c \delta t _c + c_{cal} \delta. Calorimeter Constant For Water.
From www.chegg.com
Calculate the calorimeter constant (From Lab) So for Calorimeter Constant For Water the calorimetry calculator can help you solve complex calorimetry problems. after mixing 100.0 g of water at 58.5 °c with 100.0 g of water, already in the calorimeter, at 22.8 °c, the final temperature of the. It can analyze the heat exchange between up to 3 objects. pour in a known quantity of hot water (m 1). Calorimeter Constant For Water.
From www.youtube.com
CHEMISTRY 101 Constant Pressure Calorimetry YouTube Calorimeter Constant For Water It can analyze the heat exchange between up to 3 objects. pour in a known quantity of hot water (m 1) into a calorimeter containing a known quantity of cold water (m 2) and record the equilibrium. the calorimeter constant is the net heat capacity of the bomb, the thermostat water, the bucket, and the. the calorimetry. Calorimeter Constant For Water.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Calorimeter Constant For Water the calorimeter constant for the calorimeter is 36.0 j/°c and you can obtain the specific heat capacities for gold and water from table 5.2.1. the calorimetry calculator can help you solve complex calorimetry problems. liquid water has one of the highest specific heats known. It can analyze the heat exchange between up to 3 objects. a. Calorimeter Constant For Water.
From exydnungu.blob.core.windows.net
Calorimetry A Level Biology at Melva Arnold blog Calorimeter Constant For Water liquid water has one of the highest specific heats known. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. 5.6.10 for \(t_f\), but there are two entities absorbing the heat \[ q_c +q_h=0 \\ (m_cc_c \delta t _c + c_{cal} \delta t _c) + m_hc_h \delta t_h=0 \]. Calorimeter Constant For Water.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID1875569 Calorimeter Constant For Water the calorimeter constant is the net heat capacity of the bomb, the thermostat water, the bucket, and the. pour in a known quantity of hot water (m 1) into a calorimeter containing a known quantity of cold water (m 2) and record the equilibrium. the calorimetry calculator can help you solve complex calorimetry problems. 5.6.10 for \(t_f\),. Calorimeter Constant For Water.
From slideplayer.com
Chapter 17 Thermochemistry 17.2 Measuring and Expressing ppt download Calorimeter Constant For Water liquid water has one of the highest specific heats known. the calorimeter constant for the calorimeter is 36.0 j/°c and you can obtain the specific heat capacities for gold and water from table 5.2.1. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It can analyze the. Calorimeter Constant For Water.
From www.youtube.com
050 Calorimetry YouTube Calorimeter Constant For Water 5.6.10 for \(t_f\), but there are two entities absorbing the heat \[ q_c +q_h=0 \\ (m_cc_c \delta t _c + c_{cal} \delta t _c) + m_hc_h \delta t_h=0 \] pour in a known quantity of hot water (m 1) into a calorimeter containing a known quantity of cold water (m 2) and record the equilibrium. a calorimeter is. Calorimeter Constant For Water.
From www.chegg.com
Solved Lab Data How to calculate the calorimeter constant Calorimeter Constant For Water pour in a known quantity of hot water (m 1) into a calorimeter containing a known quantity of cold water (m 2) and record the equilibrium. the calorimeter constant is the net heat capacity of the bomb, the thermostat water, the bucket, and the. after mixing 100.0 g of water at 58.5 °c with 100.0 g of. Calorimeter Constant For Water.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimeter Constant For Water a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. the calorimetry calculator can help you solve complex calorimetry problems. pour in a known quantity of hot water (m 1) into a calorimeter containing a known quantity of cold water (m 2) and record the equilibrium. after. Calorimeter Constant For Water.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses Calorimeter Constant For Water It can analyze the heat exchange between up to 3 objects. pour in a known quantity of hot water (m 1) into a calorimeter containing a known quantity of cold water (m 2) and record the equilibrium. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. the. Calorimeter Constant For Water.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation Calorimeter Constant For Water the calorimeter constant is the net heat capacity of the bomb, the thermostat water, the bucket, and the. pour in a known quantity of hot water (m 1) into a calorimeter containing a known quantity of cold water (m 2) and record the equilibrium. the calorimetry calculator can help you solve complex calorimetry problems. 5.6.10 for \(t_f\),. Calorimeter Constant For Water.
From fyopnceht.blob.core.windows.net
Definition Of Calorimetry In Physics at Patricia Cowan blog Calorimeter Constant For Water the calorimeter constant is the net heat capacity of the bomb, the thermostat water, the bucket, and the. liquid water has one of the highest specific heats known. pour in a known quantity of hot water (m 1) into a calorimeter containing a known quantity of cold water (m 2) and record the equilibrium. the calorimeter. Calorimeter Constant For Water.
From giohgceuu.blob.core.windows.net
Calorimetry What Is The Importance at Jennifer Patton blog Calorimeter Constant For Water It can analyze the heat exchange between up to 3 objects. liquid water has one of the highest specific heats known. after mixing 100.0 g of water at 58.5 °c with 100.0 g of water, already in the calorimeter, at 22.8 °c, the final temperature of the. the calorimeter constant is the net heat capacity of the. Calorimeter Constant For Water.
From www.youtube.com
Calorimeter Constant lab part 1 YouTube Calorimeter Constant For Water the calorimeter constant is the net heat capacity of the bomb, the thermostat water, the bucket, and the. It can analyze the heat exchange between up to 3 objects. the calorimeter constant for the calorimeter is 36.0 j/°c and you can obtain the specific heat capacities for gold and water from table 5.2.1. 5.6.10 for \(t_f\), but there. Calorimeter Constant For Water.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID1875569 Calorimeter Constant For Water the calorimeter constant for the calorimeter is 36.0 j/°c and you can obtain the specific heat capacities for gold and water from table 5.2.1. It can analyze the heat exchange between up to 3 objects. 5.6.10 for \(t_f\), but there are two entities absorbing the heat \[ q_c +q_h=0 \\ (m_cc_c \delta t _c + c_{cal} \delta t _c). Calorimeter Constant For Water.
From www.youtube.com
Thermochemistry 5 Determining Calorimeter Constant 6m16s YouTube Calorimeter Constant For Water after mixing 100.0 g of water at 58.5 °c with 100.0 g of water, already in the calorimeter, at 22.8 °c, the final temperature of the. the calorimeter constant is the net heat capacity of the bomb, the thermostat water, the bucket, and the. a calorimeter is a device used to measure the amount of heat involved. Calorimeter Constant For Water.
From www.youtube.com
CHEMISTRY 101 Constant volume calorimetry YouTube Calorimeter Constant For Water a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. the calorimetry calculator can help you solve complex calorimetry problems. the calorimeter constant for the calorimeter is 36.0 j/°c and you can obtain the specific heat capacities for gold and water from table 5.2.1. 5.6.10 for \(t_f\), but. Calorimeter Constant For Water.
From wisc.pb.unizin.org
Calorimetry continued Types of Calorimeters and Analyzing Heat Flow Calorimeter Constant For Water 5.6.10 for \(t_f\), but there are two entities absorbing the heat \[ q_c +q_h=0 \\ (m_cc_c \delta t _c + c_{cal} \delta t _c) + m_hc_h \delta t_h=0 \] pour in a known quantity of hot water (m 1) into a calorimeter containing a known quantity of cold water (m 2) and record the equilibrium. It can analyze the. Calorimeter Constant For Water.
From giojclhhk.blob.core.windows.net
How To Calculate Calorimeter Constant Problems at Michael Massie blog Calorimeter Constant For Water It can analyze the heat exchange between up to 3 objects. 5.6.10 for \(t_f\), but there are two entities absorbing the heat \[ q_c +q_h=0 \\ (m_cc_c \delta t _c + c_{cal} \delta t _c) + m_hc_h \delta t_h=0 \] pour in a known quantity of hot water (m 1) into a calorimeter containing a known quantity of cold. Calorimeter Constant For Water.
From www.youtube.com
Calorimetry calculation YouTube Calorimeter Constant For Water the calorimetry calculator can help you solve complex calorimetry problems. 5.6.10 for \(t_f\), but there are two entities absorbing the heat \[ q_c +q_h=0 \\ (m_cc_c \delta t _c + c_{cal} \delta t _c) + m_hc_h \delta t_h=0 \] the calorimeter constant is the net heat capacity of the bomb, the thermostat water, the bucket, and the. It. Calorimeter Constant For Water.
From saylordotorg.github.io
Calorimetry Calorimeter Constant For Water liquid water has one of the highest specific heats known. 5.6.10 for \(t_f\), but there are two entities absorbing the heat \[ q_c +q_h=0 \\ (m_cc_c \delta t _c + c_{cal} \delta t _c) + m_hc_h \delta t_h=0 \] after mixing 100.0 g of water at 58.5 °c with 100.0 g of water, already in the calorimeter, at. Calorimeter Constant For Water.
From www.slideserve.com
PPT Chapter 5 Thermochemistry PowerPoint Presentation, free download Calorimeter Constant For Water pour in a known quantity of hot water (m 1) into a calorimeter containing a known quantity of cold water (m 2) and record the equilibrium. after mixing 100.0 g of water at 58.5 °c with 100.0 g of water, already in the calorimeter, at 22.8 °c, the final temperature of the. the calorimetry calculator can help. Calorimeter Constant For Water.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimeter Constant For Water It can analyze the heat exchange between up to 3 objects. liquid water has one of the highest specific heats known. the calorimetry calculator can help you solve complex calorimetry problems. the calorimeter constant is the net heat capacity of the bomb, the thermostat water, the bucket, and the. the calorimeter constant for the calorimeter is. Calorimeter Constant For Water.