Chlorine Isotopes Fractional Abundance . There are only two stable isotopes: the table below shows the exact mass of each isotope (isotopic mass) and the percent abundance (sometimes called fractional. 35 cl, with a mass of 34.9689 amu (atomic mass units) and 37. chlorine has two naturally occurring isotopes: 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. The natural abundance of these two isotopes is observed in the mass spectrum as two peaks separated by 2 m/z with a relative intensity of \(3: The mass spectrum of chlorobenzene c 6 h 5 cl in figure \(\pageindex{1}\) clearly shows the chlorine isotope distribution at 112 m/z and 114 m/z. Once we collect the relative masses of each isotope from mass spectrometry data, we can use this information to calculate. chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the other isotope? the mass of an element shown in a periodic table or listed in a table of atomic masses is a weighted, average mass of all the isotopes present in a.
from www.chegg.com
chlorine has two naturally occurring isotopes: the mass of an element shown in a periodic table or listed in a table of atomic masses is a weighted, average mass of all the isotopes present in a. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the other isotope? the table below shows the exact mass of each isotope (isotopic mass) and the percent abundance (sometimes called fractional. The mass spectrum of chlorobenzene c 6 h 5 cl in figure \(\pageindex{1}\) clearly shows the chlorine isotope distribution at 112 m/z and 114 m/z. The natural abundance of these two isotopes is observed in the mass spectrum as two peaks separated by 2 m/z with a relative intensity of \(3: Once we collect the relative masses of each isotope from mass spectrometry data, we can use this information to calculate. There are only two stable isotopes: 35 cl, with a mass of 34.9689 amu (atomic mass units) and 37.
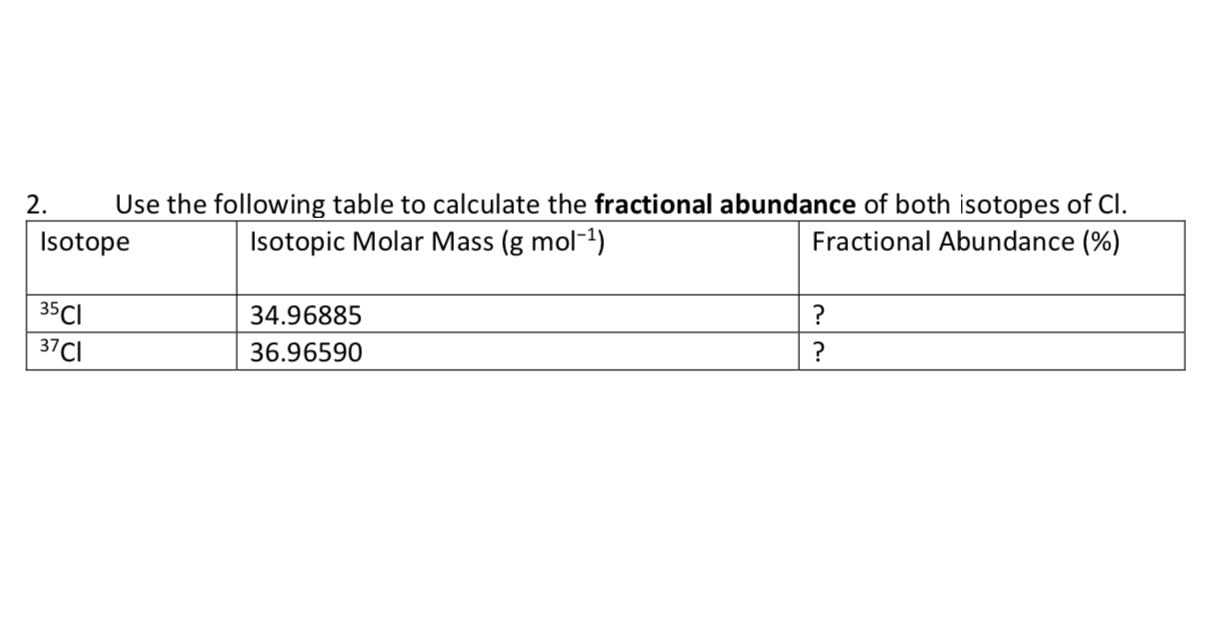
Solved 2. Use the following table to calculate the
Chlorine Isotopes Fractional Abundance The natural abundance of these two isotopes is observed in the mass spectrum as two peaks separated by 2 m/z with a relative intensity of \(3: chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the other isotope? The mass spectrum of chlorobenzene c 6 h 5 cl in figure \(\pageindex{1}\) clearly shows the chlorine isotope distribution at 112 m/z and 114 m/z. the table below shows the exact mass of each isotope (isotopic mass) and the percent abundance (sometimes called fractional. There are only two stable isotopes: chlorine has two naturally occurring isotopes: The natural abundance of these two isotopes is observed in the mass spectrum as two peaks separated by 2 m/z with a relative intensity of \(3: Once we collect the relative masses of each isotope from mass spectrometry data, we can use this information to calculate. the mass of an element shown in a periodic table or listed in a table of atomic masses is a weighted, average mass of all the isotopes present in a. 35 cl, with a mass of 34.9689 amu (atomic mass units) and 37. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl.
From www.toppr.com
A naturally occurring mixture of chlorine has the following fractional Chlorine Isotopes Fractional Abundance 35 cl, with a mass of 34.9689 amu (atomic mass units) and 37. chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. The natural abundance of these two isotopes is observed in the mass spectrum as two peaks separated by 2 m/z with a relative intensity of \(3: There are only two stable isotopes:. Chlorine Isotopes Fractional Abundance.
From www.chegg.com
Solved 2. Use the following table to calculate the Chlorine Isotopes Fractional Abundance The mass spectrum of chlorobenzene c 6 h 5 cl in figure \(\pageindex{1}\) clearly shows the chlorine isotope distribution at 112 m/z and 114 m/z. There are only two stable isotopes: the table below shows the exact mass of each isotope (isotopic mass) and the percent abundance (sometimes called fractional. chlorine has two naturally occurring isotopes: the. Chlorine Isotopes Fractional Abundance.
From ar.inspiredpencil.com
Isotopes Of Chlorine Chlorine Isotopes Fractional Abundance chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the other isotope? Once we collect the relative masses of each isotope from mass spectrometry data, we can use this information to calculate.. Chlorine Isotopes Fractional Abundance.
From www.slideserve.com
PPT 1. Atomic Structure PowerPoint Presentation, free download ID Chlorine Isotopes Fractional Abundance 35 cl, with a mass of 34.9689 amu (atomic mass units) and 37. chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the other isotope? chlorine has two naturally occurring isotopes:. Chlorine Isotopes Fractional Abundance.
From byjus.com
cl 35 and cl 37 are two isotopes of chlorine . if average atomic mass Chlorine Isotopes Fractional Abundance 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the other isotope? the table below shows the exact mass of each isotope. Chlorine Isotopes Fractional Abundance.
From www.researchgate.net
Stable isotopes of chlorine Download Scientific Diagram Chlorine Isotopes Fractional Abundance chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the other isotope? The mass spectrum of chlorobenzene c 6 h 5 cl in figure \(\pageindex{1}\) clearly shows the chlorine isotope distribution at 112 m/z and 114 m/z. The natural abundance of these two isotopes is observed in. Chlorine Isotopes Fractional Abundance.
From www.transformationtutoring.com
Percent Abundance and Average Atomic Mass Calculations Guide Chlorine Isotopes Fractional Abundance chlorine has two naturally occurring isotopes: the table below shows the exact mass of each isotope (isotopic mass) and the percent abundance (sometimes called fractional. chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the other isotope? Once we collect the relative masses of each. Chlorine Isotopes Fractional Abundance.
From www.chegg.com
Solved Please use the following table to calculate the Chlorine Isotopes Fractional Abundance chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the other isotope? the table below shows the exact mass of each isotope (isotopic mass) and the percent abundance (sometimes called fractional. chlorine has two naturally occurring isotopes: 35 cl, with a mass of 34.9689 amu. Chlorine Isotopes Fractional Abundance.
From www.alamy.com
chlorine chemical element isotopes atomic structure illustration Chlorine Isotopes Fractional Abundance The mass spectrum of chlorobenzene c 6 h 5 cl in figure \(\pageindex{1}\) clearly shows the chlorine isotope distribution at 112 m/z and 114 m/z. Once we collect the relative masses of each isotope from mass spectrometry data, we can use this information to calculate. chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl.. Chlorine Isotopes Fractional Abundance.
From slideplayer.com
Figure Title An image of the surface of the semiconductor GaAs Chlorine Isotopes Fractional Abundance chlorine has two naturally occurring isotopes: There are only two stable isotopes: The mass spectrum of chlorobenzene c 6 h 5 cl in figure \(\pageindex{1}\) clearly shows the chlorine isotope distribution at 112 m/z and 114 m/z. chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of. Chlorine Isotopes Fractional Abundance.
From www.nagwa.com
Vidéo de question Calcul de la masse atomique relative du chlore à Chlorine Isotopes Fractional Abundance 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. 35 cl, with a mass of 34.9689 amu (atomic mass units) and 37. Once we collect the relative masses of each isotope from mass spectrometry data, we can use this information to calculate. chlorine has. Chlorine Isotopes Fractional Abundance.
From www.chegg.com
Solved Chlorine has two naturally occurring isotopes, Chlorine Isotopes Fractional Abundance chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the other isotope? Once we collect the relative masses of each isotope from mass spectrometry data, we can use this information to calculate. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52. Chlorine Isotopes Fractional Abundance.
From www.showme.com
Relative abundancies of Chlorine from a mass spectrometer Science Chlorine Isotopes Fractional Abundance 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. the table below shows the exact mass of each isotope (isotopic mass) and the percent abundance (sometimes called fractional. Once we collect the relative masses of each isotope from mass spectrometry data, we can use. Chlorine Isotopes Fractional Abundance.
From www.teachoo.com
Isotopes and Isobars Definition, Uses and Difference Teachoo Chlorine Isotopes Fractional Abundance the table below shows the exact mass of each isotope (isotopic mass) and the percent abundance (sometimes called fractional. Once we collect the relative masses of each isotope from mass spectrometry data, we can use this information to calculate. 35 cl, with a mass of 34.9689 amu (atomic mass units) and 37. chlorine has 24 isotopes with mass. Chlorine Isotopes Fractional Abundance.
From www.slideserve.com
PPT Mass Spectrometry PowerPoint Presentation, free download ID4766583 Chlorine Isotopes Fractional Abundance Once we collect the relative masses of each isotope from mass spectrometry data, we can use this information to calculate. the table below shows the exact mass of each isotope (isotopic mass) and the percent abundance (sometimes called fractional. chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. chlorine has two isotopes,. Chlorine Isotopes Fractional Abundance.
From www.sciencephoto.com
Isotopes of chlorine, illustration Stock Image C028/6463 Science Chlorine Isotopes Fractional Abundance chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the other isotope? There are only two stable isotopes: the table below shows the exact mass of each isotope (isotopic mass) and. Chlorine Isotopes Fractional Abundance.
From www.toppr.com
The average relative atomic mass of chlorine is 35.45. It consists of Chlorine Isotopes Fractional Abundance The mass spectrum of chlorobenzene c 6 h 5 cl in figure \(\pageindex{1}\) clearly shows the chlorine isotope distribution at 112 m/z and 114 m/z. There are only two stable isotopes: chlorine has two naturally occurring isotopes: the table below shows the exact mass of each isotope (isotopic mass) and the percent abundance (sometimes called fractional. The natural. Chlorine Isotopes Fractional Abundance.
From slideplayer.com
32 Discovering Atomic Structure* ppt video online download Chlorine Isotopes Fractional Abundance 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. the table below shows the exact mass of each isotope (isotopic mass) and the percent abundance (sometimes called fractional. . Chlorine Isotopes Fractional Abundance.
From studiousguy.com
Chlorine (Cl) Properties & Uses StudiousGuy Chlorine Isotopes Fractional Abundance The natural abundance of these two isotopes is observed in the mass spectrum as two peaks separated by 2 m/z with a relative intensity of \(3: There are only two stable isotopes: 35 cl, with a mass of 34.9689 amu (atomic mass units) and 37. chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl.. Chlorine Isotopes Fractional Abundance.
From www.numerade.com
2.45 Naturally occurring chlorine is a mixture of the iso topes C135 Chlorine Isotopes Fractional Abundance There are only two stable isotopes: Once we collect the relative masses of each isotope from mass spectrometry data, we can use this information to calculate. The mass spectrum of chlorobenzene c 6 h 5 cl in figure \(\pageindex{1}\) clearly shows the chlorine isotope distribution at 112 m/z and 114 m/z. the mass of an element shown in a. Chlorine Isotopes Fractional Abundance.
From www.youtube.com
Atomic Mass How to Calculate Isotope Abundance YouTube Chlorine Isotopes Fractional Abundance 35 cl, with a mass of 34.9689 amu (atomic mass units) and 37. The natural abundance of these two isotopes is observed in the mass spectrum as two peaks separated by 2 m/z with a relative intensity of \(3: the mass of an element shown in a periodic table or listed in a table of atomic masses is a. Chlorine Isotopes Fractional Abundance.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID5568233 Chlorine Isotopes Fractional Abundance the table below shows the exact mass of each isotope (isotopic mass) and the percent abundance (sometimes called fractional. The natural abundance of these two isotopes is observed in the mass spectrum as two peaks separated by 2 m/z with a relative intensity of \(3: chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass. Chlorine Isotopes Fractional Abundance.
From www.slideserve.com
PPT The Chlorine Rule An Analysis of Isotope Patterns of Compounds Chlorine Isotopes Fractional Abundance 35 cl, with a mass of 34.9689 amu (atomic mass units) and 37. The mass spectrum of chlorobenzene c 6 h 5 cl in figure \(\pageindex{1}\) clearly shows the chlorine isotope distribution at 112 m/z and 114 m/z. Once we collect the relative masses of each isotope from mass spectrometry data, we can use this information to calculate. the. Chlorine Isotopes Fractional Abundance.
From www.coursehero.com
[Solved] Chlorine has two naturally occurring isotopes Chlorine 35 Chlorine Isotopes Fractional Abundance chlorine has two naturally occurring isotopes: chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. 35 cl, with a mass of 34.9689 amu (atomic mass units) and 37. Once. Chlorine Isotopes Fractional Abundance.
From pubs.acs.org
The Isotope Abundances of Chlorine from Various Sources1 Journal of Chlorine Isotopes Fractional Abundance The natural abundance of these two isotopes is observed in the mass spectrum as two peaks separated by 2 m/z with a relative intensity of \(3: 35 cl, with a mass of 34.9689 amu (atomic mass units) and 37. the table below shows the exact mass of each isotope (isotopic mass) and the percent abundance (sometimes called fractional. . Chlorine Isotopes Fractional Abundance.
From exywudvep.blob.core.windows.net
Chlorine Isotopes Amu at Hope Herzog blog Chlorine Isotopes Fractional Abundance chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. The natural abundance of these two isotopes is observed in the mass spectrum as two peaks separated by 2 m/z with a relative intensity of \(3: chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is. Chlorine Isotopes Fractional Abundance.
From byjus.com
what is abundance and how to find abundance of isotopes Chlorine Isotopes Fractional Abundance chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. 35 cl, with a mass of 34.9689 amu (atomic mass units) and 37. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. chlorine has two naturally occurring isotopes: . Chlorine Isotopes Fractional Abundance.
From www.researchgate.net
Fractional abundances of CH3OH, HC5N, and CH3CCH in the highmass Chlorine Isotopes Fractional Abundance There are only two stable isotopes: The mass spectrum of chlorobenzene c 6 h 5 cl in figure \(\pageindex{1}\) clearly shows the chlorine isotope distribution at 112 m/z and 114 m/z. chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the other isotope? The natural abundance of. Chlorine Isotopes Fractional Abundance.
From www.slideserve.com
PPT ISOTOPES PowerPoint Presentation, free download ID4551597 Chlorine Isotopes Fractional Abundance 35 cl, with a mass of 34.9689 amu (atomic mass units) and 37. chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. chlorine has two naturally occurring isotopes: the table below shows the exact mass of each isotope (isotopic mass) and the percent abundance (sometimes called fractional. The mass spectrum of chlorobenzene. Chlorine Isotopes Fractional Abundance.
From slideplayer.com
Atoms, Ions and Isotopes ! ppt download Chlorine Isotopes Fractional Abundance 35 cl, with a mass of 34.9689 amu (atomic mass units) and 37. chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. The natural abundance of these two isotopes is observed in the mass spectrum as two peaks separated by 2 m/z with a relative intensity of \(3: Once we collect the relative masses. Chlorine Isotopes Fractional Abundance.
From www.youtube.com
Calculating Isotope Abundance using Atomic Mass YouTube Chlorine Isotopes Fractional Abundance 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. Once we collect the relative masses of each isotope from mass spectrometry data, we can use this information to calculate. chlorine has two naturally occurring isotopes: 35 cl, with a mass of 34.9689 amu (atomic. Chlorine Isotopes Fractional Abundance.
From www.youtube.com
In naturally occurring neon, the fractional abundance of various Chlorine Isotopes Fractional Abundance Once we collect the relative masses of each isotope from mass spectrometry data, we can use this information to calculate. chlorine has two naturally occurring isotopes: chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. 35 cl, with a mass of 34.9689 amu (atomic mass units) and 37. chlorine has two isotopes,. Chlorine Isotopes Fractional Abundance.
From www.youtube.com
For every one 37Cl, there are three 35Cl isotopes in a sample of Chlorine Isotopes Fractional Abundance chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. chlorine has two naturally occurring isotopes: Once we collect the relative masses of each isotope from mass spectrometry data, we. Chlorine Isotopes Fractional Abundance.
From www.slideserve.com
PPT Atomic Structure PowerPoint Presentation, free download ID5850956 Chlorine Isotopes Fractional Abundance 35 cl, with a mass of 34.9689 amu (atomic mass units) and 37. chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the other isotope? Once we collect the relative masses of each isotope from mass spectrometry data, we can use this information to calculate. There are. Chlorine Isotopes Fractional Abundance.
From www.youtube.com
How to Solve for Percent Abundance of Isotopes Examples, Practice Chlorine Isotopes Fractional Abundance There are only two stable isotopes: chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. chlorine has two naturally occurring isotopes: 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. 35 cl, with a mass of 34.9689 amu. Chlorine Isotopes Fractional Abundance.