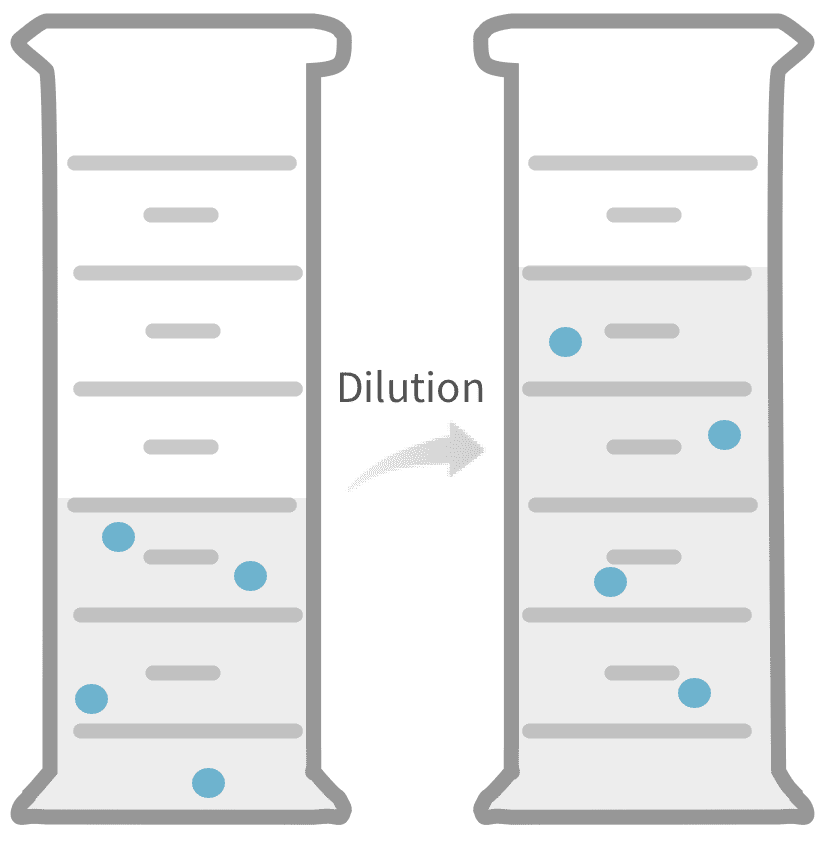
Dilution Definition Anatomy . Apply the dilution equation to calculate the final concentration, or the final volume, of a. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is a process used to lower the concentration of the original solution by adding more solvent. Concentration is the removal of solvent, which. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means before dilution, and a subscript of 2 means. dilution is the process of reducing the concentration of a solution by adding more solvent. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. This can be done in order to make. Concentration is the removal of solvent, which.
from www.wolframalpha.com
Apply the dilution equation to calculate the final concentration, or the final volume, of a. Concentration is the removal of solvent, which. This can be done in order to make. to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means before dilution, and a subscript of 2 means. dilution is the process of reducing the concentration of a solution by adding more solvent. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is a process used to lower the concentration of the original solution by adding more solvent. Concentration is the removal of solvent, which.
Dilution Calculator WolframAlpha Chemistry Solvers
Dilution Definition Anatomy dilution is the process of reducing the concentration of a solution by adding more solvent. to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means before dilution, and a subscript of 2 means. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. This can be done in order to make. dilution is a process used to lower the concentration of the original solution by adding more solvent. Concentration is the removal of solvent, which. dilution is the process of reducing the concentration of a solution by adding more solvent. Concentration is the removal of solvent, which. Apply the dilution equation to calculate the final concentration, or the final volume, of a.
From carlosgokeowen.blogspot.com
What is Dilution Dilution Definition Anatomy to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means before dilution, and a subscript of 2 means. Concentration is the removal of solvent, which. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the addition. Dilution Definition Anatomy.
From www.sliderbase.com
Strengths of Acids and Bases Making Dilutions Presentation Chemistry Dilution Definition Anatomy This can be done in order to make. Apply the dilution equation to calculate the final concentration, or the final volume, of a. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which. Concentration is the removal of solvent, which. dilution is the addition of. Dilution Definition Anatomy.
From ecampusontario.pressbooks.pub
LAB 2 Basic Techniques Introductory Bacteriology Lab Manual Dilution Definition Anatomy Concentration is the removal of solvent, which. dilution is the process of reducing the concentration of a solution by adding more solvent. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which. dilution is a process used to lower the concentration of the original. Dilution Definition Anatomy.
From www.youtube.com
DILUTIONDEFINITION AND PRACTICE PROBLEMS YouTube Dilution Definition Anatomy to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means before dilution, and a subscript of 2 means. dilution is a process used to lower the concentration of the original solution by adding more solvent. dilution is the addition of solvent, which decreases the concentration. Dilution Definition Anatomy.
From fyolbkacc.blob.core.windows.net
Dilution Definition In Chemistry at Pamela Wagner blog Dilution Definition Anatomy dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. to summarize, c stands for the concentration of the solution, v for the volume of the solution, a. Dilution Definition Anatomy.
From www.youtube.com
Dilution and Dilution Factor in Microbiology How to Calculate Dilution Definition Anatomy to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means before dilution, and a subscript of 2 means. Concentration is the removal of solvent, which. dilution is a process used to lower the concentration of the original solution by adding more solvent. dilution is the. Dilution Definition Anatomy.
From exytjuoxg.blob.core.windows.net
Dilution Of Hcl at James Payne blog Dilution Definition Anatomy This can be done in order to make. Concentration is the removal of solvent, which. Apply the dilution equation to calculate the final concentration, or the final volume, of a. dilution is the process of reducing the concentration of a solution by adding more solvent. dilution is the addition of solvent, which decreases the concentration of the solute. Dilution Definition Anatomy.
From carlosgokeowen.blogspot.com
What is Dilution Dilution Definition Anatomy Apply the dilution equation to calculate the final concentration, or the final volume, of a. dilution is a process used to lower the concentration of the original solution by adding more solvent. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. This can be done in order to make. Concentration is. Dilution Definition Anatomy.
From www.slideserve.com
PPT Lesson 18 PowerPoint Presentation, free download ID3739399 Dilution Definition Anatomy Concentration is the removal of solvent, which. This can be done in order to make. dilution is a process used to lower the concentration of the original solution by adding more solvent. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which. Apply the dilution. Dilution Definition Anatomy.
From study.com
Dilution Definition, Equation & Factors Video & Lesson Transcript Dilution Definition Anatomy dilution is a process used to lower the concentration of the original solution by adding more solvent. This can be done in order to make. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the process of reducing the concentration of a solution by adding more solvent. Concentration. Dilution Definition Anatomy.
From www.pinterest.com
Dilution ratio Wikipedia Molecular biology, Anatomy and physiology Dilution Definition Anatomy dilution is a process used to lower the concentration of the original solution by adding more solvent. dilution is the process of reducing the concentration of a solution by adding more solvent. Apply the dilution equation to calculate the final concentration, or the final volume, of a. This can be done in order to make. dilution is. Dilution Definition Anatomy.
From www.majordifferences.com
Difference between Dilution and Dilution Factor in Microbiology Dilution Definition Anatomy dilution is the addition of solvent, which decreases the concentration of the solute in the solution. to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means before dilution, and a subscript of 2 means. Concentration is the removal of solvent, which. Concentration is the removal of. Dilution Definition Anatomy.
From twinklsecondary.blog
Products of a Dilution Series A Level Biology Revision Dilution Definition Anatomy dilution is the process of reducing the concentration of a solution by adding more solvent. Concentration is the removal of solvent, which. to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means before dilution, and a subscript of 2 means. dilution is the addition of. Dilution Definition Anatomy.
From www.youtube.com
What Is Dilution? Chemistry Matters YouTube Dilution Definition Anatomy Concentration is the removal of solvent, which. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. This can be done in order to make. dilution is a process used to lower the concentration of the original solution by adding more solvent. Apply the dilution equation to calculate the final concentration, or. Dilution Definition Anatomy.
From www.studypool.com
SOLUTION Dilution chemistry Studypool Dilution Definition Anatomy dilution is the addition of solvent, which decreases the concentration of the solute in the solution. This can be done in order to make. Apply the dilution equation to calculate the final concentration, or the final volume, of a. Concentration is the removal of solvent, which. dilution is a process used to lower the concentration of the original. Dilution Definition Anatomy.
From www.slideserve.com
PPT Lesson 18 PowerPoint Presentation, free download ID3739399 Dilution Definition Anatomy Concentration is the removal of solvent, which. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. This can be done in order to make. dilution is a process used to lower the concentration of. Dilution Definition Anatomy.
From www.medicine.mcgill.ca
Serial Dilutions Dilution Definition Anatomy Concentration is the removal of solvent, which. dilution is a process used to lower the concentration of the original solution by adding more solvent. This can be done in order to make. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the addition of solvent, which decreases the. Dilution Definition Anatomy.
From www.slideshare.net
Serial dilution Dilution Definition Anatomy dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the process of reducing the concentration. Dilution Definition Anatomy.
From www.slideserve.com
PPT Dilution and Evaporation PowerPoint Presentation, free download Dilution Definition Anatomy Concentration is the removal of solvent, which. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means before dilution, and a subscript of 2 means. dilution is a process. Dilution Definition Anatomy.
From exyoyarnx.blob.core.windows.net
Ideal Dilute Solution Example at Tina Huckins blog Dilution Definition Anatomy dilution is a process used to lower the concentration of the original solution by adding more solvent. This can be done in order to make. dilution is the process of reducing the concentration of a solution by adding more solvent. Concentration is the removal of solvent, which. dilution is the addition of solvent, which decreases the concentration. Dilution Definition Anatomy.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube Dilution Definition Anatomy to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means before dilution, and a subscript of 2 means. Apply the dilution equation to calculate the final concentration, or the final volume, of a. dilution is the addition of solvent, which decreases the concentration of the solute. Dilution Definition Anatomy.
From testbook.com
Dilution Learn its Definition, Formula, Method & Importance Dilution Definition Anatomy dilution is the addition of solvent, which decreases the concentration of the solute in the solution. to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means before dilution, and a subscript of 2 means. dilution is a process used to lower the concentration of the. Dilution Definition Anatomy.
From gioartpaa.blob.core.windows.net
What Are Dilutions Chemistry at Julian Smith blog Dilution Definition Anatomy dilution is a process used to lower the concentration of the original solution by adding more solvent. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. This can be done in order to make. dilution is the addition of solvent, which decreases the concentration of the solute in the solution.. Dilution Definition Anatomy.
From giowqjocn.blob.core.windows.net
Dilution Formula Define at John Vaughn blog Dilution Definition Anatomy dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which. to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means before dilution, and a subscript of 2 means. dilution is the addition. Dilution Definition Anatomy.
From gioartpaa.blob.core.windows.net
What Are Dilutions Chemistry at Julian Smith blog Dilution Definition Anatomy dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is a process used to lower the concentration of the original solution by adding more solvent. to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means before dilution,. Dilution Definition Anatomy.
From fyolbkacc.blob.core.windows.net
Dilution Definition In Chemistry at Pamela Wagner blog Dilution Definition Anatomy dilution is a process used to lower the concentration of the original solution by adding more solvent. dilution is the process of reducing the concentration of a solution by adding more solvent. Concentration is the removal of solvent, which. Apply the dilution equation to calculate the final concentration, or the final volume, of a. to summarize, c. Dilution Definition Anatomy.
From gioartpaa.blob.core.windows.net
What Are Dilutions Chemistry at Julian Smith blog Dilution Definition Anatomy This can be done in order to make. to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means before dilution, and a subscript of 2 means. Apply the dilution equation to calculate the final concentration, or the final volume, of a. dilution is a process used. Dilution Definition Anatomy.
From www.etsy.com
Dilution Guide Serial & Normal Clinical Laboratory Dilutions MLT/MLS Dilution Definition Anatomy Concentration is the removal of solvent, which. Apply the dilution equation to calculate the final concentration, or the final volume, of a. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is a process used to lower the concentration of the original solution by adding more solvent. to summarize,. Dilution Definition Anatomy.
From alevelchemistry.co.uk
Dilution Facts, Summary & Definition Chemistry Revision Dilution Definition Anatomy This can be done in order to make. dilution is the process of reducing the concentration of a solution by adding more solvent. Apply the dilution equation to calculate the final concentration, or the final volume, of a. dilution is a process used to lower the concentration of the original solution by adding more solvent. dilution is. Dilution Definition Anatomy.
From giokewoed.blob.core.windows.net
How Does Dilution Work Chemistry at Margaret Gamble blog Dilution Definition Anatomy Concentration is the removal of solvent, which. Apply the dilution equation to calculate the final concentration, or the final volume, of a. dilution is a process used to lower the concentration of the original solution by adding more solvent. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. This can be. Dilution Definition Anatomy.
From www.wolframalpha.com
Dilution Calculator WolframAlpha Chemistry Solvers Dilution Definition Anatomy dilution is a process used to lower the concentration of the original solution by adding more solvent. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which. Concentration is the removal of solvent, which. dilution is the addition of solvent, which decreases the concentration. Dilution Definition Anatomy.
From www.osmosis.org
Molarity and dilutions Vídeo, Anatomía & Definición Osmosis Dilution Definition Anatomy Concentration is the removal of solvent, which. dilution is the process of reducing the concentration of a solution by adding more solvent. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Apply the dilution equation to calculate the final concentration, or the final volume, of a. dilution is the addition. Dilution Definition Anatomy.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilution Definition Anatomy This can be done in order to make. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Concentration is the removal of solvent, which. dilution is the process of reducing the concentration of a. Dilution Definition Anatomy.
From www.aquaportail.com
Dilution définition et explications AquaPortail Dilution Definition Anatomy Concentration is the removal of solvent, which. This can be done in order to make. dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Apply the dilution equation to calculate the final concentration, or the final volume, of a. dilution is the addition of solvent, which decreases the concentration of the. Dilution Definition Anatomy.
From carlosgokeowen.blogspot.com
What is Dilution Dilution Definition Anatomy dilution is the addition of solvent, which decreases the concentration of the solute in the solution. to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means before dilution, and a subscript of 2 means. dilution is the addition of solvent, which decreases the concentration of. Dilution Definition Anatomy.