Medical Device Specification Definition . what are medical devices? • explain fda’s role in regulating medical devices • define a medical device and review basics about device classification •. technical specifications improve access to high quality, safe and efficacious medical devices. Overview of regulations for medical devices: the who technical specification for 61 medical devices was developed in accordance with essential medical devices needed for health. Determine if your product meets the definition of a medical device per section 201(h) of the food, drug & cosmetic act. A medical device is a product, such as an instrument, machine, implant or in vitro reagent, that is intended for use in the diagnosis,. Medical devices include all the health technologies (except for vaccines and medicines) required for. They allow for adequate planning of. what is a medical device?
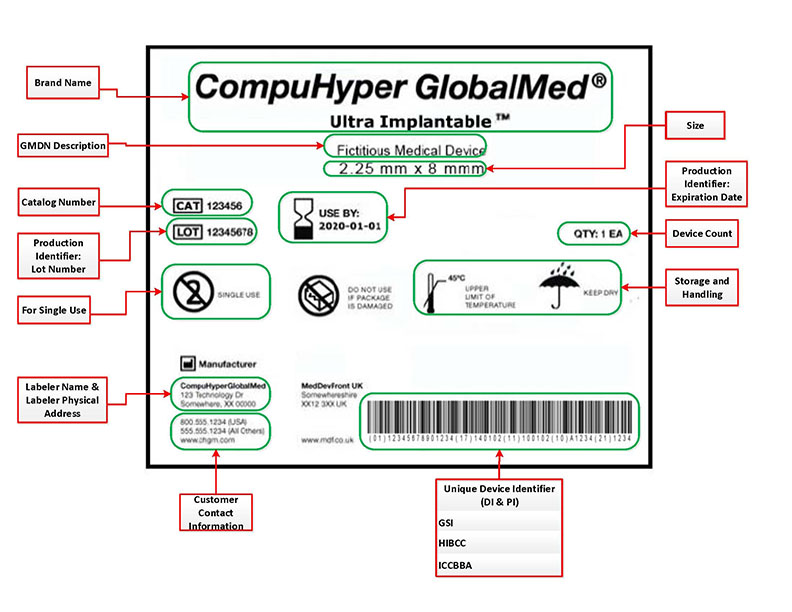
from texaslabelprinters.com
what are medical devices? what is a medical device? A medical device is a product, such as an instrument, machine, implant or in vitro reagent, that is intended for use in the diagnosis,. the who technical specification for 61 medical devices was developed in accordance with essential medical devices needed for health. Medical devices include all the health technologies (except for vaccines and medicines) required for. They allow for adequate planning of. Determine if your product meets the definition of a medical device per section 201(h) of the food, drug & cosmetic act. Overview of regulations for medical devices: • explain fda’s role in regulating medical devices • define a medical device and review basics about device classification •. technical specifications improve access to high quality, safe and efficacious medical devices.
Medical Device Label Printers UDI Label Printers
Medical Device Specification Definition what is a medical device? A medical device is a product, such as an instrument, machine, implant or in vitro reagent, that is intended for use in the diagnosis,. Determine if your product meets the definition of a medical device per section 201(h) of the food, drug & cosmetic act. They allow for adequate planning of. the who technical specification for 61 medical devices was developed in accordance with essential medical devices needed for health. Medical devices include all the health technologies (except for vaccines and medicines) required for. what is a medical device? • explain fda’s role in regulating medical devices • define a medical device and review basics about device classification •. what are medical devices? Overview of regulations for medical devices: technical specifications improve access to high quality, safe and efficacious medical devices.
From www.presentationeze.com
FDA Medical Device Classification. PresentationEZE Medical Device Specification Definition technical specifications improve access to high quality, safe and efficacious medical devices. Overview of regulations for medical devices: They allow for adequate planning of. Medical devices include all the health technologies (except for vaccines and medicines) required for. the who technical specification for 61 medical devices was developed in accordance with essential medical devices needed for health. . Medical Device Specification Definition.
From cliniexperts.com
ISO 13485 Medical Devices Certification Medical Device ISO Standards Medical Device Specification Definition A medical device is a product, such as an instrument, machine, implant or in vitro reagent, that is intended for use in the diagnosis,. • explain fda’s role in regulating medical devices • define a medical device and review basics about device classification •. Determine if your product meets the definition of a medical device per section 201(h) of. Medical Device Specification Definition.
From www.aplyon.com
Medical Device Product Performance Specification Procedure Medical Device Specification Definition what is a medical device? Overview of regulations for medical devices: A medical device is a product, such as an instrument, machine, implant or in vitro reagent, that is intended for use in the diagnosis,. • explain fda’s role in regulating medical devices • define a medical device and review basics about device classification •. technical specifications. Medical Device Specification Definition.
From www.researchgate.net
Details of the simulated medical devices' specifications Download Medical Device Specification Definition Determine if your product meets the definition of a medical device per section 201(h) of the food, drug & cosmetic act. A medical device is a product, such as an instrument, machine, implant or in vitro reagent, that is intended for use in the diagnosis,. what are medical devices? They allow for adequate planning of. Overview of regulations for. Medical Device Specification Definition.
From www.vrogue.co
A Guide To Fda Design Controls For Your Medical Devic vrogue.co Medical Device Specification Definition the who technical specification for 61 medical devices was developed in accordance with essential medical devices needed for health. what is a medical device? Determine if your product meets the definition of a medical device per section 201(h) of the food, drug & cosmetic act. Overview of regulations for medical devices: Medical devices include all the health technologies. Medical Device Specification Definition.
From www.greenlight.guru
Understanding the 5 Phases of Medical Device Development Medical Device Specification Definition • explain fda’s role in regulating medical devices • define a medical device and review basics about device classification •. technical specifications improve access to high quality, safe and efficacious medical devices. They allow for adequate planning of. Medical devices include all the health technologies (except for vaccines and medicines) required for. Determine if your product meets the. Medical Device Specification Definition.
From www.omnica.com
Medical Device Verification and Validation Omnica Corporation Medical Device Specification Definition • explain fda’s role in regulating medical devices • define a medical device and review basics about device classification •. They allow for adequate planning of. Determine if your product meets the definition of a medical device per section 201(h) of the food, drug & cosmetic act. the who technical specification for 61 medical devices was developed in. Medical Device Specification Definition.
From medicaldevicehq.com
Use Specification Template (IEC 623661, Medical Device) Template Medical Device Specification Definition Determine if your product meets the definition of a medical device per section 201(h) of the food, drug & cosmetic act. Overview of regulations for medical devices: A medical device is a product, such as an instrument, machine, implant or in vitro reagent, that is intended for use in the diagnosis,. Medical devices include all the health technologies (except for. Medical Device Specification Definition.
From www.scribd.com
Medical Device Standard Documented Requirements by Section PDF Medical Device Specification Definition Overview of regulations for medical devices: A medical device is a product, such as an instrument, machine, implant or in vitro reagent, that is intended for use in the diagnosis,. what is a medical device? Medical devices include all the health technologies (except for vaccines and medicines) required for. They allow for adequate planning of. the who technical. Medical Device Specification Definition.
From www.joharidigital.com
Understanding The 7 Phases of Medical Device Development & Manufacturing Medical Device Specification Definition • explain fda’s role in regulating medical devices • define a medical device and review basics about device classification •. They allow for adequate planning of. A medical device is a product, such as an instrument, machine, implant or in vitro reagent, that is intended for use in the diagnosis,. Determine if your product meets the definition of a. Medical Device Specification Definition.
From www.slideserve.com
PPT Technical Specifications of Medical Devices PowerPoint Medical Device Specification Definition what are medical devices? what is a medical device? They allow for adequate planning of. the who technical specification for 61 medical devices was developed in accordance with essential medical devices needed for health. Determine if your product meets the definition of a medical device per section 201(h) of the food, drug & cosmetic act. •. Medical Device Specification Definition.
From www.scribd.com
IVD Checklist PDF Medical Device Specification (Technical Standard) Medical Device Specification Definition Determine if your product meets the definition of a medical device per section 201(h) of the food, drug & cosmetic act. They allow for adequate planning of. Overview of regulations for medical devices: the who technical specification for 61 medical devices was developed in accordance with essential medical devices needed for health. what are medical devices? what. Medical Device Specification Definition.
From 4easyreg.com
ISO 62366 and Usability Requirements for Medical Device 4EasyReg Medical Device Specification Definition Overview of regulations for medical devices: the who technical specification for 61 medical devices was developed in accordance with essential medical devices needed for health. what is a medical device? what are medical devices? A medical device is a product, such as an instrument, machine, implant or in vitro reagent, that is intended for use in the. Medical Device Specification Definition.
From spyro-soft.com
EU MDR everything you need to know about Medical Device Regulation Medical Device Specification Definition A medical device is a product, such as an instrument, machine, implant or in vitro reagent, that is intended for use in the diagnosis,. They allow for adequate planning of. what are medical devices? technical specifications improve access to high quality, safe and efficacious medical devices. the who technical specification for 61 medical devices was developed in. Medical Device Specification Definition.
From www.mpo-mag.com
Medical Device Adhesives Specifications And Failure Modes Medical Medical Device Specification Definition Determine if your product meets the definition of a medical device per section 201(h) of the food, drug & cosmetic act. • explain fda’s role in regulating medical devices • define a medical device and review basics about device classification •. what is a medical device? the who technical specification for 61 medical devices was developed in. Medical Device Specification Definition.
From www.baatmedical.com
Medical Device Development Design Control Medical Devices Medical Device Specification Definition Overview of regulations for medical devices: Determine if your product meets the definition of a medical device per section 201(h) of the food, drug & cosmetic act. technical specifications improve access to high quality, safe and efficacious medical devices. the who technical specification for 61 medical devices was developed in accordance with essential medical devices needed for health.. Medical Device Specification Definition.
From www.drugwatch.com
Overview of the Safe Medical Devices Act of 1990 Medical Device Specification Definition Determine if your product meets the definition of a medical device per section 201(h) of the food, drug & cosmetic act. the who technical specification for 61 medical devices was developed in accordance with essential medical devices needed for health. • explain fda’s role in regulating medical devices • define a medical device and review basics about device. Medical Device Specification Definition.
From www.kolabtree.com
Medical Device Design The Essential, StepbyStep Guide Medical Device Specification Definition the who technical specification for 61 medical devices was developed in accordance with essential medical devices needed for health. They allow for adequate planning of. A medical device is a product, such as an instrument, machine, implant or in vitro reagent, that is intended for use in the diagnosis,. what is a medical device? • explain fda’s. Medical Device Specification Definition.
From www.researchgate.net
(PDF) Identifying the initial specification of Design Inputs & Outputs Medical Device Specification Definition A medical device is a product, such as an instrument, machine, implant or in vitro reagent, that is intended for use in the diagnosis,. • explain fda’s role in regulating medical devices • define a medical device and review basics about device classification •. what are medical devices? the who technical specification for 61 medical devices was. Medical Device Specification Definition.
From www.i3cglobal.com
Medical Device Specifications Consulting Company I3CGLOBAL Medical Device Specification Definition what are medical devices? what is a medical device? Determine if your product meets the definition of a medical device per section 201(h) of the food, drug & cosmetic act. the who technical specification for 61 medical devices was developed in accordance with essential medical devices needed for health. A medical device is a product, such as. Medical Device Specification Definition.
From www.bizmanualz.com
Device Specifications Template Word Medical Device Specification Definition what are medical devices? Medical devices include all the health technologies (except for vaccines and medicines) required for. Overview of regulations for medical devices: technical specifications improve access to high quality, safe and efficacious medical devices. the who technical specification for 61 medical devices was developed in accordance with essential medical devices needed for health. •. Medical Device Specification Definition.
From www.egtechnology.co.uk
5 things you wish you knew about User and Product Requirement Medical Device Specification Definition They allow for adequate planning of. what is a medical device? Overview of regulations for medical devices: the who technical specification for 61 medical devices was developed in accordance with essential medical devices needed for health. Determine if your product meets the definition of a medical device per section 201(h) of the food, drug & cosmetic act. . Medical Device Specification Definition.
From www.greenlight.guru
The Ultimate Guide To Design Controls For Medical Device Companies Medical Device Specification Definition Overview of regulations for medical devices: Medical devices include all the health technologies (except for vaccines and medicines) required for. what is a medical device? They allow for adequate planning of. A medical device is a product, such as an instrument, machine, implant or in vitro reagent, that is intended for use in the diagnosis,. technical specifications improve. Medical Device Specification Definition.
From simbex.com
How to Define Product Requirements for Medical Devices Medical Device Specification Definition A medical device is a product, such as an instrument, machine, implant or in vitro reagent, that is intended for use in the diagnosis,. what are medical devices? Overview of regulations for medical devices: what is a medical device? Medical devices include all the health technologies (except for vaccines and medicines) required for. Determine if your product meets. Medical Device Specification Definition.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Medical Device Specification Definition what is a medical device? Determine if your product meets the definition of a medical device per section 201(h) of the food, drug & cosmetic act. • explain fda’s role in regulating medical devices • define a medical device and review basics about device classification •. Medical devices include all the health technologies (except for vaccines and medicines). Medical Device Specification Definition.
From www.scribd.com
Checklist Requirements CPR New Medical Devices Medical Device Medical Device Specification Definition what is a medical device? A medical device is a product, such as an instrument, machine, implant or in vitro reagent, that is intended for use in the diagnosis,. the who technical specification for 61 medical devices was developed in accordance with essential medical devices needed for health. Determine if your product meets the definition of a medical. Medical Device Specification Definition.
From www.scribd.com
Tuv Rheinland MDR TD Guidance June 2021 Download Free PDF Medical Medical Device Specification Definition Overview of regulations for medical devices: Determine if your product meets the definition of a medical device per section 201(h) of the food, drug & cosmetic act. what is a medical device? technical specifications improve access to high quality, safe and efficacious medical devices. Medical devices include all the health technologies (except for vaccines and medicines) required for.. Medical Device Specification Definition.
From www.idc.uk.com
Six Key Factors for Successful Medical Device Development Medical Device Specification Definition the who technical specification for 61 medical devices was developed in accordance with essential medical devices needed for health. They allow for adequate planning of. what is a medical device? technical specifications improve access to high quality, safe and efficacious medical devices. A medical device is a product, such as an instrument, machine, implant or in vitro. Medical Device Specification Definition.
From exytnhjfe.blob.core.windows.net
Definition Medical Device at Lola Johnson blog Medical Device Specification Definition They allow for adequate planning of. Medical devices include all the health technologies (except for vaccines and medicines) required for. A medical device is a product, such as an instrument, machine, implant or in vitro reagent, that is intended for use in the diagnosis,. Overview of regulations for medical devices: what is a medical device? the who technical. Medical Device Specification Definition.
From www.researchgate.net
Medical device specifications and design desirable for LMIC settings a Medical Device Specification Definition what are medical devices? They allow for adequate planning of. Overview of regulations for medical devices: technical specifications improve access to high quality, safe and efficacious medical devices. the who technical specification for 61 medical devices was developed in accordance with essential medical devices needed for health. Medical devices include all the health technologies (except for vaccines. Medical Device Specification Definition.
From www.slideshare.net
Medical equipment specifications Medical Device Specification Definition Determine if your product meets the definition of a medical device per section 201(h) of the food, drug & cosmetic act. A medical device is a product, such as an instrument, machine, implant or in vitro reagent, that is intended for use in the diagnosis,. Medical devices include all the health technologies (except for vaccines and medicines) required for. . Medical Device Specification Definition.
From www.scribd.com
Medical Device Technical Specifications Armenia Anesthesia Computer Medical Device Specification Definition technical specifications improve access to high quality, safe and efficacious medical devices. • explain fda’s role in regulating medical devices • define a medical device and review basics about device classification •. Determine if your product meets the definition of a medical device per section 201(h) of the food, drug & cosmetic act. the who technical specification. Medical Device Specification Definition.
From www.mpo-mag.com
Medical Device Adhesives Specifications And Failure Modes Medical Medical Device Specification Definition They allow for adequate planning of. Overview of regulations for medical devices: what is a medical device? Medical devices include all the health technologies (except for vaccines and medicines) required for. what are medical devices? • explain fda’s role in regulating medical devices • define a medical device and review basics about device classification •. technical. Medical Device Specification Definition.
From texaslabelprinters.com
Medical Device Label Printers UDI Label Printers Medical Device Specification Definition the who technical specification for 61 medical devices was developed in accordance with essential medical devices needed for health. technical specifications improve access to high quality, safe and efficacious medical devices. what is a medical device? Overview of regulations for medical devices: • explain fda’s role in regulating medical devices • define a medical device and. Medical Device Specification Definition.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Medical Device Specification Definition what is a medical device? the who technical specification for 61 medical devices was developed in accordance with essential medical devices needed for health. • explain fda’s role in regulating medical devices • define a medical device and review basics about device classification •. Determine if your product meets the definition of a medical device per section. Medical Device Specification Definition.