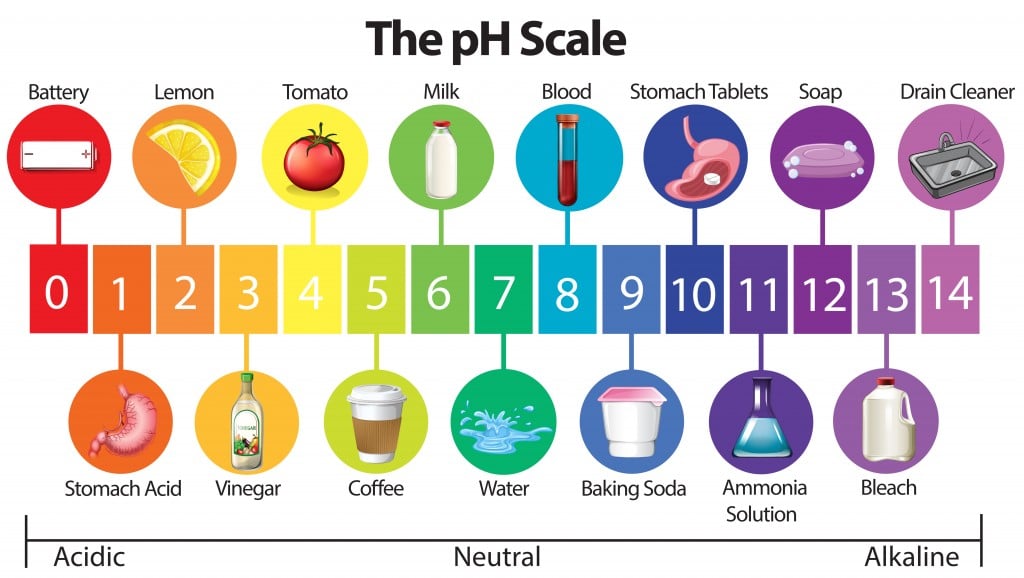
What Does Ph Actually Measure . The term, widely used in chemistry, biology, and agronomy, translates the. Ph is defined as the negative log of hydrogen ion concentration. Because it is based on a logarithmic. Ph, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. Ph is a measure of how acidic or basic a substance is, based on the concentration of hydrogen ions. Learn how to use the ph formula, how to calculate ph, and how to measure ph with. In fact, the term ph originates from latin and. It takes a numerical value between 0 and 14 that measures the relative amount of free hydrogen and hydroxyl ions in water. Ph is a measure of how acidic or basic a substance is. Learn how the scale works, what substances have different ph. Ph describes how acidic or basic a solution is. It can be used to describe the relative acidity (or basicity) of a solution. Whether an aqueous solution reacts as an acid or a base depends on its hydrogen ion (h +) content. The ph scale measures how acidic or basic a solution is, based on its ability to donate or accept protons.
from www.scienceabc.com
Ph, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. Ph is a measure of how acidic or basic a substance is. In fact, the term ph originates from latin and. Ph is defined as the negative log of hydrogen ion concentration. Ph describes how acidic or basic a solution is. Ph is a measure of how acidic or basic a substance is, based on the concentration of hydrogen ions. Because it is based on a logarithmic. The ph scale measures how acidic or basic a solution is, based on its ability to donate or accept protons. Learn how to use the ph formula, how to calculate ph, and how to measure ph with. Learn how the scale works, what substances have different ph.
Why Does The pH Scale Range From 0 To 14? Can It Go Beyond That Range?
What Does Ph Actually Measure It can be used to describe the relative acidity (or basicity) of a solution. In fact, the term ph originates from latin and. Ph is a measure of how acidic or basic a substance is. Learn how the scale works, what substances have different ph. Learn how to use the ph formula, how to calculate ph, and how to measure ph with. Because it is based on a logarithmic. The ph scale measures how acidic or basic a solution is, based on its ability to donate or accept protons. Ph describes how acidic or basic a solution is. Ph is a measure of how acidic or basic a substance is, based on the concentration of hydrogen ions. Ph, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. Ph is defined as the negative log of hydrogen ion concentration. The term, widely used in chemistry, biology, and agronomy, translates the. It takes a numerical value between 0 and 14 that measures the relative amount of free hydrogen and hydroxyl ions in water. It can be used to describe the relative acidity (or basicity) of a solution. Whether an aqueous solution reacts as an acid or a base depends on its hydrogen ion (h +) content.
From blog.havells.com
Right pH Level in Drinking Water How Essential? Havells India Blog What Does Ph Actually Measure Ph is a measure of how acidic or basic a substance is. Learn how to use the ph formula, how to calculate ph, and how to measure ph with. Ph describes how acidic or basic a solution is. In fact, the term ph originates from latin and. Whether an aqueous solution reacts as an acid or a base depends on. What Does Ph Actually Measure.
From psiberg.com
The pH Scale of Acid and Bases PSIBERG What Does Ph Actually Measure Whether an aqueous solution reacts as an acid or a base depends on its hydrogen ion (h +) content. Because it is based on a logarithmic. Learn how the scale works, what substances have different ph. Ph is a measure of how acidic or basic a substance is. Ph is defined as the negative log of hydrogen ion concentration. In. What Does Ph Actually Measure.
From depositphotos.com
Ph scale. infographic acidbase balance. scale for chemical analysis What Does Ph Actually Measure Whether an aqueous solution reacts as an acid or a base depends on its hydrogen ion (h +) content. Ph is a measure of how acidic or basic a substance is. Learn how the scale works, what substances have different ph. Ph is defined as the negative log of hydrogen ion concentration. The term, widely used in chemistry, biology, and. What Does Ph Actually Measure.
From www.bartleby.com
What Does the pH Scale Measure? Free Expert Q&A bartleby What Does Ph Actually Measure Ph is a measure of how acidic or basic a substance is. Ph, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. Learn how the scale works, what substances have different ph. Ph is a measure of how acidic or basic a substance is, based on the concentration of hydrogen ions. Because it is based on. What Does Ph Actually Measure.
From www.fierceelectronics.com
Eight Ways To Improve pH Measurement Reliability Fierce Electronics What Does Ph Actually Measure Ph describes how acidic or basic a solution is. Because it is based on a logarithmic. In fact, the term ph originates from latin and. Ph is a measure of how acidic or basic a substance is, based on the concentration of hydrogen ions. Ph, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. It takes. What Does Ph Actually Measure.
From www.chemswot.com
Definition of pH scale in Chemistry What Does Ph Actually Measure Learn how the scale works, what substances have different ph. Whether an aqueous solution reacts as an acid or a base depends on its hydrogen ion (h +) content. Ph is a measure of how acidic or basic a substance is. Ph is defined as the negative log of hydrogen ion concentration. Learn how to use the ph formula, how. What Does Ph Actually Measure.
From getwellstaywellathome.com
Using pH as a Health Monitor Get Well Stay Well At Home What Does Ph Actually Measure Ph is a measure of how acidic or basic a substance is. It takes a numerical value between 0 and 14 that measures the relative amount of free hydrogen and hydroxyl ions in water. Ph describes how acidic or basic a solution is. In fact, the term ph originates from latin and. Learn how the scale works, what substances have. What Does Ph Actually Measure.
From ournutritionkitchen.com
Think fast what’s your pH? What Does Ph Actually Measure It can be used to describe the relative acidity (or basicity) of a solution. The term, widely used in chemistry, biology, and agronomy, translates the. Learn how to use the ph formula, how to calculate ph, and how to measure ph with. Because it is based on a logarithmic. Learn how the scale works, what substances have different ph. Ph,. What Does Ph Actually Measure.
From www.medicalnewstoday.com
What to know about the pH of water What Does Ph Actually Measure Learn how the scale works, what substances have different ph. Because it is based on a logarithmic. The ph scale measures how acidic or basic a solution is, based on its ability to donate or accept protons. In fact, the term ph originates from latin and. Learn how to use the ph formula, how to calculate ph, and how to. What Does Ph Actually Measure.
From www.alamy.com
Measurement of the pH scale, pressure gauge, infographics. pH is a What Does Ph Actually Measure It can be used to describe the relative acidity (or basicity) of a solution. Ph, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. In fact, the term ph originates from latin and. Ph describes how acidic or basic a solution is. Whether an aqueous solution reacts as an acid or a base depends on its. What Does Ph Actually Measure.
From www.toppr.com
pH Definition, Formula, Meaning, Applications and More What Does Ph Actually Measure The term, widely used in chemistry, biology, and agronomy, translates the. It takes a numerical value between 0 and 14 that measures the relative amount of free hydrogen and hydroxyl ions in water. Ph describes how acidic or basic a solution is. Ph is a measure of how acidic or basic a substance is, based on the concentration of hydrogen. What Does Ph Actually Measure.
From www.vecteezy.com
The pH Scale diagram on white background 2988621 Vector Art at Vecteezy What Does Ph Actually Measure Ph describes how acidic or basic a solution is. Because it is based on a logarithmic. Ph is a measure of how acidic or basic a substance is. Learn how the scale works, what substances have different ph. It can be used to describe the relative acidity (or basicity) of a solution. It takes a numerical value between 0 and. What Does Ph Actually Measure.
From www.dreamstime.com
PH Scale Indicator Chart Diagram Acidic Alkaline Measure. PH Analysis What Does Ph Actually Measure Learn how the scale works, what substances have different ph. Learn how to use the ph formula, how to calculate ph, and how to measure ph with. Ph, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. It can be used to describe the relative acidity (or basicity) of a solution. Ph is defined as the. What Does Ph Actually Measure.
From www.danapointwater.com
pH Dana Point Water What Does Ph Actually Measure Because it is based on a logarithmic. Ph describes how acidic or basic a solution is. Ph is a measure of how acidic or basic a substance is. Learn how the scale works, what substances have different ph. Ph is defined as the negative log of hydrogen ion concentration. Ph is a measure of how acidic or basic a substance. What Does Ph Actually Measure.
From www.expii.com
What Is pH? — Definition & Overview Expii What Does Ph Actually Measure In fact, the term ph originates from latin and. Learn how to use the ph formula, how to calculate ph, and how to measure ph with. Ph describes how acidic or basic a solution is. Learn how the scale works, what substances have different ph. The term, widely used in chemistry, biology, and agronomy, translates the. Ph is a measure. What Does Ph Actually Measure.
From www.slideserve.com
PPT pH and Buffers PowerPoint Presentation, free download ID2948821 What Does Ph Actually Measure In fact, the term ph originates from latin and. Ph is defined as the negative log of hydrogen ion concentration. Whether an aqueous solution reacts as an acid or a base depends on its hydrogen ion (h +) content. The ph scale measures how acidic or basic a solution is, based on its ability to donate or accept protons. Ph. What Does Ph Actually Measure.
From dynamixinc.com
PH Alkalinity Mixing With Reaction Times Dynamix Agitators What Does Ph Actually Measure Because it is based on a logarithmic. It takes a numerical value between 0 and 14 that measures the relative amount of free hydrogen and hydroxyl ions in water. Whether an aqueous solution reacts as an acid or a base depends on its hydrogen ion (h +) content. Ph describes how acidic or basic a solution is. Ph is a. What Does Ph Actually Measure.
From sciencenotes.org
The pH Scale of Common Chemicals What Does Ph Actually Measure Ph, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. Because it is based on a logarithmic. Whether an aqueous solution reacts as an acid or a base depends on its hydrogen ion (h +) content. Learn how to use the ph formula, how to calculate ph, and how to measure ph with. The term, widely. What Does Ph Actually Measure.
From www.youtube.com
MB.1.2. Ph scale (HSC biology) YouTube What Does Ph Actually Measure Ph, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. Whether an aqueous solution reacts as an acid or a base depends on its hydrogen ion (h +) content. The term, widely used in chemistry, biology, and agronomy, translates the. Because it is based on a logarithmic. Ph is a measure of how acidic or basic. What Does Ph Actually Measure.
From www.hamiltoncompany.com
The pH Scale Process Analytics What Does Ph Actually Measure The term, widely used in chemistry, biology, and agronomy, translates the. Learn how to use the ph formula, how to calculate ph, and how to measure ph with. Ph describes how acidic or basic a solution is. Because it is based on a logarithmic. Learn how the scale works, what substances have different ph. Ph is a measure of how. What Does Ph Actually Measure.
From www.slideserve.com
PPT Biology I What is pH? PowerPoint Presentation, free download ID What Does Ph Actually Measure The ph scale measures how acidic or basic a solution is, based on its ability to donate or accept protons. Whether an aqueous solution reacts as an acid or a base depends on its hydrogen ion (h +) content. It can be used to describe the relative acidity (or basicity) of a solution. Ph describes how acidic or basic a. What Does Ph Actually Measure.
From www.pmel.noaa.gov
The pH scale by numbers What Does Ph Actually Measure Learn how to use the ph formula, how to calculate ph, and how to measure ph with. Because it is based on a logarithmic. Ph is a measure of how acidic or basic a substance is. It takes a numerical value between 0 and 14 that measures the relative amount of free hydrogen and hydroxyl ions in water. In fact,. What Does Ph Actually Measure.
From www.sciencelearn.org.nz
pH scale — Science Learning Hub What Does Ph Actually Measure Ph, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. Ph is a measure of how acidic or basic a substance is. It takes a numerical value between 0 and 14 that measures the relative amount of free hydrogen and hydroxyl ions in water. In fact, the term ph originates from latin and. Whether an aqueous. What Does Ph Actually Measure.
From www.thoughtco.com
pH Definition and Equation in Chemistry What Does Ph Actually Measure It takes a numerical value between 0 and 14 that measures the relative amount of free hydrogen and hydroxyl ions in water. Ph is a measure of how acidic or basic a substance is. Whether an aqueous solution reacts as an acid or a base depends on its hydrogen ion (h +) content. Learn how to use the ph formula,. What Does Ph Actually Measure.
From www.sciencenewsforstudents.org
Explainer What the pH scale tells us Science News for Students What Does Ph Actually Measure Ph is a measure of how acidic or basic a substance is, based on the concentration of hydrogen ions. It takes a numerical value between 0 and 14 that measures the relative amount of free hydrogen and hydroxyl ions in water. Ph, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. Ph is a measure of. What Does Ph Actually Measure.
From www.vecteezy.com
The ph scale diagram 589313 Vector Art at Vecteezy What Does Ph Actually Measure It can be used to describe the relative acidity (or basicity) of a solution. Learn how the scale works, what substances have different ph. It takes a numerical value between 0 and 14 that measures the relative amount of free hydrogen and hydroxyl ions in water. Ph, quantitative measure of the acidity or basicity of aqueous or other liquid solutions.. What Does Ph Actually Measure.
From www.scienceabc.com
Why Does The pH Scale Range From 0 To 14? Can It Go Beyond That Range? What Does Ph Actually Measure Ph, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. Ph is a measure of how acidic or basic a substance is. Because it is based on a logarithmic. The ph scale measures how acidic or basic a solution is, based on its ability to donate or accept protons. It takes a numerical value between 0. What Does Ph Actually Measure.
From naturalbiohealth.com
How Learning the pH Scale Can Create a More Balanced Diet Natural Bio What Does Ph Actually Measure Ph is defined as the negative log of hydrogen ion concentration. Learn how to use the ph formula, how to calculate ph, and how to measure ph with. It can be used to describe the relative acidity (or basicity) of a solution. In fact, the term ph originates from latin and. The ph scale measures how acidic or basic a. What Does Ph Actually Measure.
From blog.biomall.in
How does a pH meter work? Biomall Blog What Does Ph Actually Measure Ph is a measure of how acidic or basic a substance is, based on the concentration of hydrogen ions. Learn how to use the ph formula, how to calculate ph, and how to measure ph with. In fact, the term ph originates from latin and. Because it is based on a logarithmic. It can be used to describe the relative. What Does Ph Actually Measure.
From www.youtube.com
Using a pH Meter Everything You Need to Know About pH YouTube What Does Ph Actually Measure It can be used to describe the relative acidity (or basicity) of a solution. The ph scale measures how acidic or basic a solution is, based on its ability to donate or accept protons. Learn how the scale works, what substances have different ph. Ph is defined as the negative log of hydrogen ion concentration. Ph is a measure of. What Does Ph Actually Measure.
From www.mometrix.com
pH Overview (Chemistry Review Video) What Does Ph Actually Measure Learn how the scale works, what substances have different ph. Whether an aqueous solution reacts as an acid or a base depends on its hydrogen ion (h +) content. It can be used to describe the relative acidity (or basicity) of a solution. Ph, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. Ph is a. What Does Ph Actually Measure.
From www.worksheetsplanet.com
What is PH Definition What Does Ph Actually Measure In fact, the term ph originates from latin and. Ph describes how acidic or basic a solution is. Learn how to use the ph formula, how to calculate ph, and how to measure ph with. It takes a numerical value between 0 and 14 that measures the relative amount of free hydrogen and hydroxyl ions in water. Learn how the. What Does Ph Actually Measure.
From www.slideserve.com
PPT pH and Buffers PowerPoint Presentation, free download ID2948821 What Does Ph Actually Measure Ph is defined as the negative log of hydrogen ion concentration. Ph is a measure of how acidic or basic a substance is, based on the concentration of hydrogen ions. The ph scale measures how acidic or basic a solution is, based on its ability to donate or accept protons. Ph is a measure of how acidic or basic a. What Does Ph Actually Measure.
From animalia-life.club
Ph Scale Horizontal What Does Ph Actually Measure Ph, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. Learn how the scale works, what substances have different ph. Ph is a measure of how acidic or basic a substance is. Ph is defined as the negative log of hydrogen ion concentration. The ph scale measures how acidic or basic a solution is, based on. What Does Ph Actually Measure.