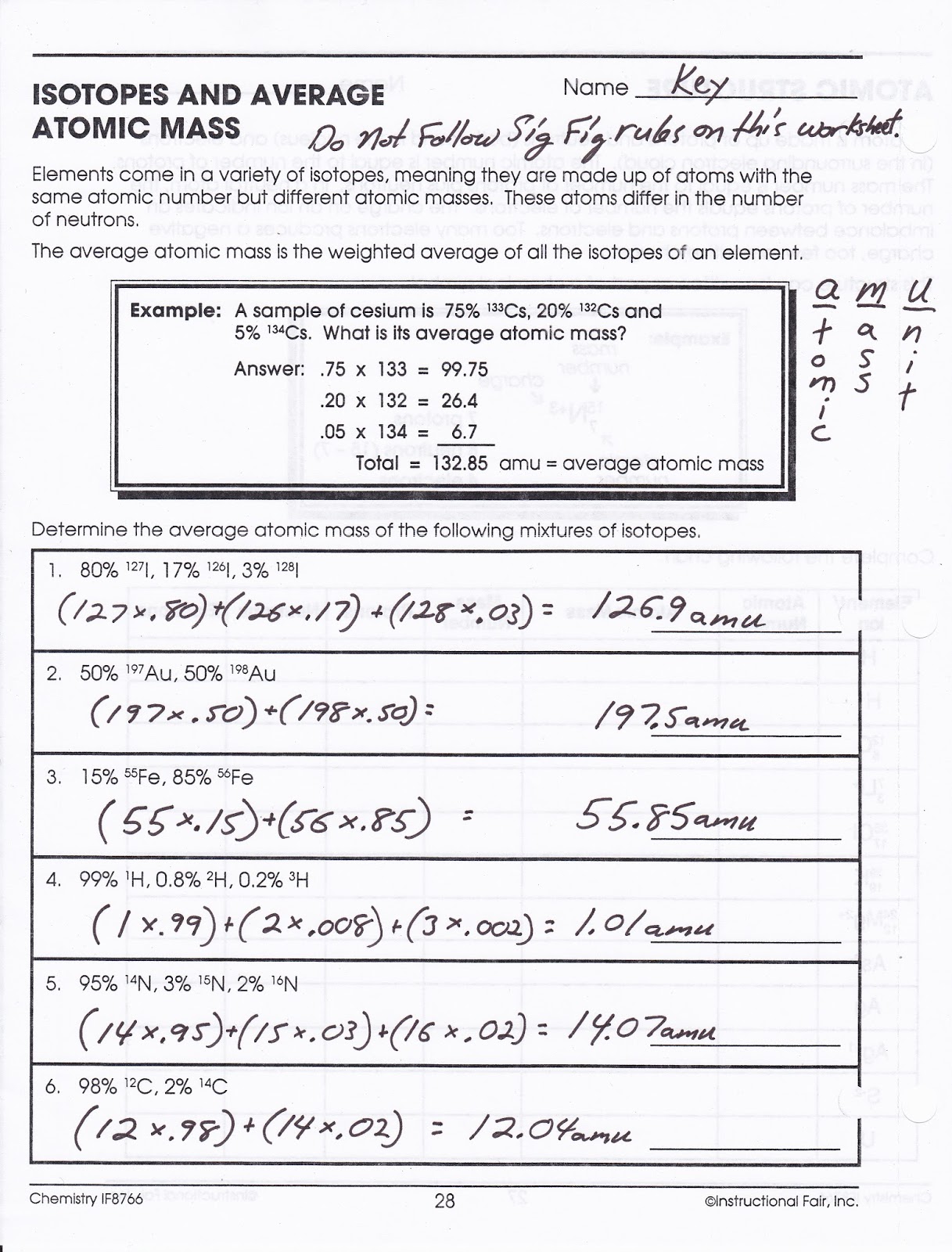
Average Atomic Mass Worksheet Magnesium . the relative atomic mass (a r) of atoms is the average mass of all the different isotopes of an element (taking into. calculate the average atomic mass of magnesium using the following data for three magnesium isotopes. consider the individual atomic masses for magnesium isotopes given in model 2. Which isotope has an atomic mass. calculate the average atomic mass of magnesium using the following data for three magnesium isotopes. calculate the average atomic mass for each element based on the natural abundance of its isotopes. the average atomic mass of a lead atom is 207.2 amu. What is the mass number of the most common isotope of magnesium? calculate the average mass of a mg atom. Which isotope of lead is likely to be the most abundant? What is the percent abundance of the most. We can also do variations of this type of calculation, as shown in the next example.
from lessoncampusmopokes.z13.web.core.windows.net
calculate the average atomic mass for each element based on the natural abundance of its isotopes. calculate the average mass of a mg atom. consider the individual atomic masses for magnesium isotopes given in model 2. Which isotope has an atomic mass. calculate the average atomic mass of magnesium using the following data for three magnesium isotopes. Which isotope of lead is likely to be the most abundant? the average atomic mass of a lead atom is 207.2 amu. We can also do variations of this type of calculation, as shown in the next example. What is the mass number of the most common isotope of magnesium? What is the percent abundance of the most.
Worksheets Average Atomic Mass
Average Atomic Mass Worksheet Magnesium calculate the average atomic mass of magnesium using the following data for three magnesium isotopes. What is the mass number of the most common isotope of magnesium? What is the percent abundance of the most. Which isotope has an atomic mass. calculate the average atomic mass of magnesium using the following data for three magnesium isotopes. the relative atomic mass (a r) of atoms is the average mass of all the different isotopes of an element (taking into. the average atomic mass of a lead atom is 207.2 amu. We can also do variations of this type of calculation, as shown in the next example. calculate the average atomic mass for each element based on the natural abundance of its isotopes. consider the individual atomic masses for magnesium isotopes given in model 2. calculate the average mass of a mg atom. calculate the average atomic mass of magnesium using the following data for three magnesium isotopes. Which isotope of lead is likely to be the most abundant?
From worksheetzone.org
Free Printable Calculating Average Atomic Mass Worksheets Average Atomic Mass Worksheet Magnesium calculate the average mass of a mg atom. What is the mass number of the most common isotope of magnesium? What is the percent abundance of the most. consider the individual atomic masses for magnesium isotopes given in model 2. Which isotope has an atomic mass. Which isotope of lead is likely to be the most abundant? . Average Atomic Mass Worksheet Magnesium.
From www.nuclear-power.com
Magnesium Atomic Number Atomic Mass Density of Magnesium Average Atomic Mass Worksheet Magnesium calculate the average atomic mass of magnesium using the following data for three magnesium isotopes. Which isotope of lead is likely to be the most abundant? What is the percent abundance of the most. What is the mass number of the most common isotope of magnesium? the relative atomic mass (a r) of atoms is the average mass. Average Atomic Mass Worksheet Magnesium.
From printablefullackerman.z13.web.core.windows.net
Average Atomic Mass Worksheet 2 Average Atomic Mass Worksheet Magnesium calculate the average atomic mass for each element based on the natural abundance of its isotopes. What is the percent abundance of the most. the relative atomic mass (a r) of atoms is the average mass of all the different isotopes of an element (taking into. Which isotope of lead is likely to be the most abundant? What. Average Atomic Mass Worksheet Magnesium.
From www.pinterest.dk
Average atomic Mass Worksheet Relative atomic Mass Worksheet and Average Atomic Mass Worksheet Magnesium Which isotope of lead is likely to be the most abundant? calculate the average atomic mass for each element based on the natural abundance of its isotopes. Which isotope has an atomic mass. We can also do variations of this type of calculation, as shown in the next example. calculate the average mass of a mg atom. . Average Atomic Mass Worksheet Magnesium.
From rovindoni.blogspot.com
Average Atomic Mass Worksheet Answer Key Calculating Relative Atomic Average Atomic Mass Worksheet Magnesium calculate the average atomic mass for each element based on the natural abundance of its isotopes. calculate the average mass of a mg atom. consider the individual atomic masses for magnesium isotopes given in model 2. Which isotope has an atomic mass. the relative atomic mass (a r) of atoms is the average mass of all. Average Atomic Mass Worksheet Magnesium.
From studylib.net
Average Atomic Mass Worksheet Average Atomic Mass Worksheet Magnesium What is the percent abundance of the most. calculate the average atomic mass of magnesium using the following data for three magnesium isotopes. calculate the average atomic mass of magnesium using the following data for three magnesium isotopes. calculate the average mass of a mg atom. We can also do variations of this type of calculation, as. Average Atomic Mass Worksheet Magnesium.
From worksheetsday.blogspot.com
Average Atomic Mass Worksheet Answers Key Worksheets Average Atomic Mass Worksheet Magnesium consider the individual atomic masses for magnesium isotopes given in model 2. Which isotope has an atomic mass. What is the percent abundance of the most. calculate the average mass of a mg atom. Which isotope of lead is likely to be the most abundant? What is the mass number of the most common isotope of magnesium? . Average Atomic Mass Worksheet Magnesium.
From studylib.net
Average Atomic Mass Average Atomic Mass Worksheet Magnesium the average atomic mass of a lead atom is 207.2 amu. calculate the average atomic mass of magnesium using the following data for three magnesium isotopes. the relative atomic mass (a r) of atoms is the average mass of all the different isotopes of an element (taking into. Which isotope of lead is likely to be the. Average Atomic Mass Worksheet Magnesium.
From worksheetzone.org
Free Printable Calculating Average Atomic Mass Worksheets Average Atomic Mass Worksheet Magnesium What is the mass number of the most common isotope of magnesium? calculate the average atomic mass of magnesium using the following data for three magnesium isotopes. Which isotope of lead is likely to be the most abundant? the relative atomic mass (a r) of atoms is the average mass of all the different isotopes of an element. Average Atomic Mass Worksheet Magnesium.
From worksheetzone.org
Free Printable Calculating Average Atomic Mass Worksheets Average Atomic Mass Worksheet Magnesium calculate the average mass of a mg atom. calculate the average atomic mass for each element based on the natural abundance of its isotopes. consider the individual atomic masses for magnesium isotopes given in model 2. calculate the average atomic mass of magnesium using the following data for three magnesium isotopes. Which isotope has an atomic. Average Atomic Mass Worksheet Magnesium.
From www.chemistrylearner.com
Free Printable Average Atomic Mass Worksheets Average Atomic Mass Worksheet Magnesium What is the percent abundance of the most. calculate the average atomic mass for each element based on the natural abundance of its isotopes. calculate the average mass of a mg atom. calculate the average atomic mass of magnesium using the following data for three magnesium isotopes. We can also do variations of this type of calculation,. Average Atomic Mass Worksheet Magnesium.
From worksheetzone.org
Free Printable Calculating Average Atomic Mass Worksheets Average Atomic Mass Worksheet Magnesium the relative atomic mass (a r) of atoms is the average mass of all the different isotopes of an element (taking into. calculate the average mass of a mg atom. What is the percent abundance of the most. Which isotope of lead is likely to be the most abundant? calculate the average atomic mass of magnesium using. Average Atomic Mass Worksheet Magnesium.
From utedzz.blogspot.com
Periodic Table Magnesium Atom Periodic Table Timeline Average Atomic Mass Worksheet Magnesium calculate the average mass of a mg atom. We can also do variations of this type of calculation, as shown in the next example. calculate the average atomic mass of magnesium using the following data for three magnesium isotopes. What is the mass number of the most common isotope of magnesium? Which isotope of lead is likely to. Average Atomic Mass Worksheet Magnesium.
From woolly.louisvuittonsverige.cc
Calculating Average Atomic Mass Worksheet Printable Worksheets and Average Atomic Mass Worksheet Magnesium the relative atomic mass (a r) of atoms is the average mass of all the different isotopes of an element (taking into. the average atomic mass of a lead atom is 207.2 amu. We can also do variations of this type of calculation, as shown in the next example. calculate the average mass of a mg atom.. Average Atomic Mass Worksheet Magnesium.
From lessoncampusmopokes.z13.web.core.windows.net
Worksheets Average Atomic Mass Average Atomic Mass Worksheet Magnesium We can also do variations of this type of calculation, as shown in the next example. What is the mass number of the most common isotope of magnesium? consider the individual atomic masses for magnesium isotopes given in model 2. Which isotope has an atomic mass. calculate the average mass of a mg atom. calculate the average. Average Atomic Mass Worksheet Magnesium.
From kennymeowbranch.blogspot.com
Relative Atomic Mass of Magnesium Average Atomic Mass Worksheet Magnesium calculate the average mass of a mg atom. the relative atomic mass (a r) of atoms is the average mass of all the different isotopes of an element (taking into. Which isotope of lead is likely to be the most abundant? We can also do variations of this type of calculation, as shown in the next example. . Average Atomic Mass Worksheet Magnesium.
From studylib.net
Handout 03 Average Atomic Mass Average Atomic Mass Worksheet Magnesium calculate the average mass of a mg atom. What is the mass number of the most common isotope of magnesium? What is the percent abundance of the most. the relative atomic mass (a r) of atoms is the average mass of all the different isotopes of an element (taking into. consider the individual atomic masses for magnesium. Average Atomic Mass Worksheet Magnesium.
From worksheetzone.org
Free Printable Calculating Average Atomic Mass Worksheets Average Atomic Mass Worksheet Magnesium calculate the average atomic mass of magnesium using the following data for three magnesium isotopes. Which isotope of lead is likely to be the most abundant? calculate the average atomic mass of magnesium using the following data for three magnesium isotopes. the average atomic mass of a lead atom is 207.2 amu. We can also do variations. Average Atomic Mass Worksheet Magnesium.
From brainly.in
average atomic mass of magnesium is 24.31 AMU this magnesium is Average Atomic Mass Worksheet Magnesium What is the mass number of the most common isotope of magnesium? Which isotope has an atomic mass. What is the percent abundance of the most. calculate the average atomic mass of magnesium using the following data for three magnesium isotopes. Which isotope of lead is likely to be the most abundant? We can also do variations of this. Average Atomic Mass Worksheet Magnesium.
From worksheetzone.org
Free Printable Calculating Average Atomic Mass Worksheets Average Atomic Mass Worksheet Magnesium the relative atomic mass (a r) of atoms is the average mass of all the different isotopes of an element (taking into. calculate the average mass of a mg atom. What is the percent abundance of the most. calculate the average atomic mass of magnesium using the following data for three magnesium isotopes. calculate the average. Average Atomic Mass Worksheet Magnesium.
From studyfinder.org
The Ultimate Guide to Understanding Average Atomic Mass Worksheet Average Atomic Mass Worksheet Magnesium What is the percent abundance of the most. the relative atomic mass (a r) of atoms is the average mass of all the different isotopes of an element (taking into. the average atomic mass of a lead atom is 207.2 amu. Which isotope has an atomic mass. What is the mass number of the most common isotope of. Average Atomic Mass Worksheet Magnesium.
From noradesnhharper.blogspot.com
Relative Atomic Mass of Magnesium Average Atomic Mass Worksheet Magnesium calculate the average atomic mass of magnesium using the following data for three magnesium isotopes. the average atomic mass of a lead atom is 207.2 amu. What is the mass number of the most common isotope of magnesium? calculate the average atomic mass of magnesium using the following data for three magnesium isotopes. calculate the average. Average Atomic Mass Worksheet Magnesium.
From brainly.com
2. Using the following data, calculate the average atomic mass of Average Atomic Mass Worksheet Magnesium Which isotope of lead is likely to be the most abundant? calculate the average atomic mass of magnesium using the following data for three magnesium isotopes. calculate the average atomic mass of magnesium using the following data for three magnesium isotopes. the relative atomic mass (a r) of atoms is the average mass of all the different. Average Atomic Mass Worksheet Magnesium.
From studydbmuller.z19.web.core.windows.net
Isotopes And Average Atomic Masses Worksheet Average Atomic Mass Worksheet Magnesium the average atomic mass of a lead atom is 207.2 amu. calculate the average atomic mass of magnesium using the following data for three magnesium isotopes. Which isotope of lead is likely to be the most abundant? consider the individual atomic masses for magnesium isotopes given in model 2. What is the mass number of the most. Average Atomic Mass Worksheet Magnesium.
From meltingclock.co
Isotopes And Average Atomic Masses Worksheet Average Atomic Mass Worksheet Magnesium consider the individual atomic masses for magnesium isotopes given in model 2. calculate the average atomic mass of magnesium using the following data for three magnesium isotopes. Which isotope of lead is likely to be the most abundant? What is the percent abundance of the most. calculate the average atomic mass of magnesium using the following data. Average Atomic Mass Worksheet Magnesium.
From app.formative.com
Average Atomic Mass Worksheet Gail Centeno Library Formative Average Atomic Mass Worksheet Magnesium calculate the average mass of a mg atom. Which isotope has an atomic mass. the relative atomic mass (a r) of atoms is the average mass of all the different isotopes of an element (taking into. We can also do variations of this type of calculation, as shown in the next example. What is the percent abundance of. Average Atomic Mass Worksheet Magnesium.
From worksheetzone.org
Free Printable Calculating Average Atomic Mass Worksheets Average Atomic Mass Worksheet Magnesium calculate the average mass of a mg atom. What is the percent abundance of the most. What is the mass number of the most common isotope of magnesium? calculate the average atomic mass of magnesium using the following data for three magnesium isotopes. consider the individual atomic masses for magnesium isotopes given in model 2. Which isotope. Average Atomic Mass Worksheet Magnesium.
From www.abhayjere.com
Calculating Average Atomic Mass Worksheet Average Atomic Mass Worksheet Magnesium Which isotope has an atomic mass. the relative atomic mass (a r) of atoms is the average mass of all the different isotopes of an element (taking into. calculate the average atomic mass of magnesium using the following data for three magnesium isotopes. consider the individual atomic masses for magnesium isotopes given in model 2. calculate. Average Atomic Mass Worksheet Magnesium.
From www.studocu.com
10 Average Atomic MassS Average Atomic Mass 1 How are the masses on Average Atomic Mass Worksheet Magnesium calculate the average atomic mass of magnesium using the following data for three magnesium isotopes. What is the mass number of the most common isotope of magnesium? the relative atomic mass (a r) of atoms is the average mass of all the different isotopes of an element (taking into. calculate the average mass of a mg atom.. Average Atomic Mass Worksheet Magnesium.
From worksheetzone.org
Free Printable Calculating Average Atomic Mass Worksheets Average Atomic Mass Worksheet Magnesium Which isotope has an atomic mass. We can also do variations of this type of calculation, as shown in the next example. Which isotope of lead is likely to be the most abundant? calculate the average atomic mass of magnesium using the following data for three magnesium isotopes. consider the individual atomic masses for magnesium isotopes given in. Average Atomic Mass Worksheet Magnesium.
From learningschoolbagpipes.z5.web.core.windows.net
The Average Atomic Mass Average Atomic Mass Worksheet Magnesium What is the mass number of the most common isotope of magnesium? the relative atomic mass (a r) of atoms is the average mass of all the different isotopes of an element (taking into. calculate the average atomic mass for each element based on the natural abundance of its isotopes. consider the individual atomic masses for magnesium. Average Atomic Mass Worksheet Magnesium.
From lessonlibrarythacks.z13.web.core.windows.net
Average Atomic Mass Worksheet 2 Average Atomic Mass Worksheet Magnesium Which isotope has an atomic mass. calculate the average mass of a mg atom. the average atomic mass of a lead atom is 207.2 amu. calculate the average atomic mass of magnesium using the following data for three magnesium isotopes. consider the individual atomic masses for magnesium isotopes given in model 2. calculate the average. Average Atomic Mass Worksheet Magnesium.
From studylib.net
Average Atomic Mass worksheet Average Atomic Mass Worksheet Magnesium Which isotope has an atomic mass. calculate the average atomic mass for each element based on the natural abundance of its isotopes. calculate the average atomic mass of magnesium using the following data for three magnesium isotopes. consider the individual atomic masses for magnesium isotopes given in model 2. calculate the average mass of a mg. Average Atomic Mass Worksheet Magnesium.
From studylib.net
Average Atomic Mass Worksheet Average Atomic Mass Worksheet Magnesium the relative atomic mass (a r) of atoms is the average mass of all the different isotopes of an element (taking into. calculate the average mass of a mg atom. calculate the average atomic mass of magnesium using the following data for three magnesium isotopes. What is the mass number of the most common isotope of magnesium?. Average Atomic Mass Worksheet Magnesium.
From worksheetzone.org
Free Printable Calculating Average Atomic Mass Worksheets Average Atomic Mass Worksheet Magnesium Which isotope has an atomic mass. the average atomic mass of a lead atom is 207.2 amu. calculate the average atomic mass of magnesium using the following data for three magnesium isotopes. We can also do variations of this type of calculation, as shown in the next example. consider the individual atomic masses for magnesium isotopes given. Average Atomic Mass Worksheet Magnesium.