Copper Electronegativity . Electronegativity is a chemical property that describes how well an atom. 119 rows find the electronegativity values of all elements in a table, including copper (1.90). Shorthand electron configuration of copper: [ar] 3d 10 4s 1. The electronegativity of copper is: Electronegativity, symbol χ, is a chemical property that describes the tendency of an atom to attract electrons towards this atom. Electronegativity of copper is 1.9. Compute answers using wolfram's breakthrough technology & knowledgebase, relied on by. In general, an atom’s electronegativity is affected by both its atomic number and the. The first scale of electronegativity was developed by linus pauling and on his scale copper has a value of 1.90 on a scale running from from. Have a question about using wolfram|alpha?
from www.numerade.com
[ar] 3d 10 4s 1. Compute answers using wolfram's breakthrough technology & knowledgebase, relied on by. 119 rows find the electronegativity values of all elements in a table, including copper (1.90). Electronegativity of copper is 1.9. The first scale of electronegativity was developed by linus pauling and on his scale copper has a value of 1.90 on a scale running from from. Shorthand electron configuration of copper: In general, an atom’s electronegativity is affected by both its atomic number and the. Electronegativity is a chemical property that describes how well an atom. Electronegativity, symbol χ, is a chemical property that describes the tendency of an atom to attract electrons towards this atom. Have a question about using wolfram|alpha?
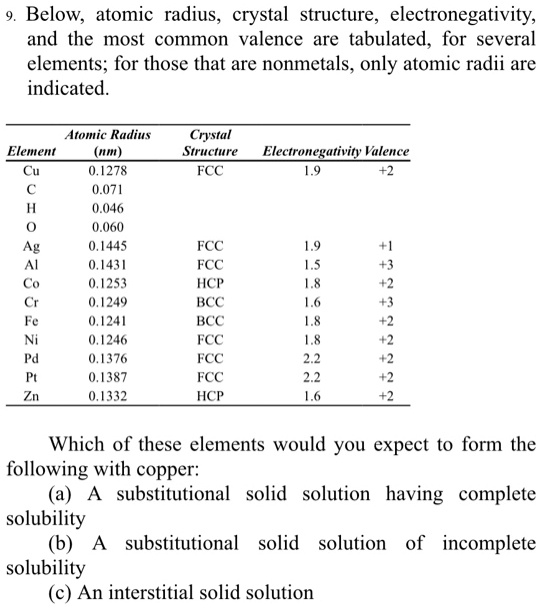
SOLVED Below; atomic radius, crystal structure, electronegativity, and
Copper Electronegativity Electronegativity of copper is 1.9. In general, an atom’s electronegativity is affected by both its atomic number and the. The electronegativity of copper is: Compute answers using wolfram's breakthrough technology & knowledgebase, relied on by. Shorthand electron configuration of copper: [ar] 3d 10 4s 1. Electronegativity of copper is 1.9. Have a question about using wolfram|alpha? The first scale of electronegativity was developed by linus pauling and on his scale copper has a value of 1.90 on a scale running from from. Electronegativity, symbol χ, is a chemical property that describes the tendency of an atom to attract electrons towards this atom. Electronegativity is a chemical property that describes how well an atom. 119 rows find the electronegativity values of all elements in a table, including copper (1.90).
From www.numerade.com
SOLVED Below; atomic radius, crystal structure, electronegativity, and Copper Electronegativity Electronegativity of copper is 1.9. 119 rows find the electronegativity values of all elements in a table, including copper (1.90). Electronegativity is a chemical property that describes how well an atom. Have a question about using wolfram|alpha? Compute answers using wolfram's breakthrough technology & knowledgebase, relied on by. Electronegativity, symbol χ, is a chemical property that describes the tendency of. Copper Electronegativity.
From sciencenotes.org
List of Electronegativity Values of the Elements Copper Electronegativity 119 rows find the electronegativity values of all elements in a table, including copper (1.90). Have a question about using wolfram|alpha? The first scale of electronegativity was developed by linus pauling and on his scale copper has a value of 1.90 on a scale running from from. The electronegativity of copper is: In general, an atom’s electronegativity is affected by. Copper Electronegativity.
From www.nanowerk.com
Electronegativity explained Copper Electronegativity Compute answers using wolfram's breakthrough technology & knowledgebase, relied on by. [ar] 3d 10 4s 1. In general, an atom’s electronegativity is affected by both its atomic number and the. Electronegativity, symbol χ, is a chemical property that describes the tendency of an atom to attract electrons towards this atom. Electronegativity is a chemical property that describes how well an. Copper Electronegativity.
From www.vrogue.co
Printable Electronegativity Periodic Table Electroneg vrogue.co Copper Electronegativity Shorthand electron configuration of copper: The electronegativity of copper is: Electronegativity, symbol χ, is a chemical property that describes the tendency of an atom to attract electrons towards this atom. Compute answers using wolfram's breakthrough technology & knowledgebase, relied on by. Electronegativity of copper is 1.9. The first scale of electronegativity was developed by linus pauling and on his scale. Copper Electronegativity.
From southernplantationstylefurniture.blogspot.com
The Periodic Table Trend Of Electronegativity Follows What Pattern Copper Electronegativity Electronegativity of copper is 1.9. The first scale of electronegativity was developed by linus pauling and on his scale copper has a value of 1.90 on a scale running from from. Compute answers using wolfram's breakthrough technology & knowledgebase, relied on by. [ar] 3d 10 4s 1. Have a question about using wolfram|alpha? Electronegativity, symbol χ, is a chemical property. Copper Electronegativity.
From www.nuclear-power.com
Copper Electron Affinity Electronegativity Ionization Energy of Copper Electronegativity Electronegativity, symbol χ, is a chemical property that describes the tendency of an atom to attract electrons towards this atom. Compute answers using wolfram's breakthrough technology & knowledgebase, relied on by. The electronegativity of copper is: The first scale of electronegativity was developed by linus pauling and on his scale copper has a value of 1.90 on a scale running. Copper Electronegativity.
From www.geeksforgeeks.org
Electronegativity Definition, Meaning, Periodic Trends, Examples Copper Electronegativity The first scale of electronegativity was developed by linus pauling and on his scale copper has a value of 1.90 on a scale running from from. The electronegativity of copper is: Electronegativity, symbol χ, is a chemical property that describes the tendency of an atom to attract electrons towards this atom. Electronegativity of copper is 1.9. [ar] 3d 10 4s. Copper Electronegativity.
From www.animalia-life.club
Electronegativity Periodic Table 3d Copper Electronegativity Electronegativity is a chemical property that describes how well an atom. [ar] 3d 10 4s 1. The first scale of electronegativity was developed by linus pauling and on his scale copper has a value of 1.90 on a scale running from from. Electronegativity of copper is 1.9. Have a question about using wolfram|alpha? Shorthand electron configuration of copper: Electronegativity, symbol. Copper Electronegativity.
From chemistry.com.pk
Electronegativity and Electronegativity Chart in PDF Copper Electronegativity Electronegativity, symbol χ, is a chemical property that describes the tendency of an atom to attract electrons towards this atom. 119 rows find the electronegativity values of all elements in a table, including copper (1.90). The electronegativity of copper is: In general, an atom’s electronegativity is affected by both its atomic number and the. The first scale of electronegativity was. Copper Electronegativity.
From dona.tompkinscountystructuralracism.org
Electronegativity Values Chart Understanding The Chemistry Of Elements Copper Electronegativity Shorthand electron configuration of copper: Electronegativity of copper is 1.9. In general, an atom’s electronegativity is affected by both its atomic number and the. The electronegativity of copper is: The first scale of electronegativity was developed by linus pauling and on his scale copper has a value of 1.90 on a scale running from from. [ar] 3d 10 4s 1.. Copper Electronegativity.
From sciencenotes.org
Electronegativity Definition and Trend Copper Electronegativity [ar] 3d 10 4s 1. Electronegativity is a chemical property that describes how well an atom. The first scale of electronegativity was developed by linus pauling and on his scale copper has a value of 1.90 on a scale running from from. Shorthand electron configuration of copper: Have a question about using wolfram|alpha? Electronegativity of copper is 1.9. The electronegativity. Copper Electronegativity.
From www.animalia-life.club
Electronegativity Energy Periodic Table Copper Electronegativity 119 rows find the electronegativity values of all elements in a table, including copper (1.90). [ar] 3d 10 4s 1. The electronegativity of copper is: In general, an atom’s electronegativity is affected by both its atomic number and the. Compute answers using wolfram's breakthrough technology & knowledgebase, relied on by. Electronegativity is a chemical property that describes how well an. Copper Electronegativity.
From utedzz.blogspot.com
Printable Periodic Table With Electronegativity Values Periodic Table Copper Electronegativity Electronegativity of copper is 1.9. Compute answers using wolfram's breakthrough technology & knowledgebase, relied on by. The first scale of electronegativity was developed by linus pauling and on his scale copper has a value of 1.90 on a scale running from from. Electronegativity, symbol χ, is a chemical property that describes the tendency of an atom to attract electrons towards. Copper Electronegativity.
From www.alamy.com
Copper chemical element with first ionization energy, atomic mass and Copper Electronegativity Shorthand electron configuration of copper: [ar] 3d 10 4s 1. 119 rows find the electronegativity values of all elements in a table, including copper (1.90). Electronegativity of copper is 1.9. Have a question about using wolfram|alpha? Electronegativity is a chemical property that describes how well an atom. Compute answers using wolfram's breakthrough technology & knowledgebase, relied on by. The first. Copper Electronegativity.
From www.shutterstock.com
Electronegativity Table Elements Color Code Stock Vector (Royalty Free Copper Electronegativity Electronegativity of copper is 1.9. Compute answers using wolfram's breakthrough technology & knowledgebase, relied on by. The electronegativity of copper is: Electronegativity, symbol χ, is a chemical property that describes the tendency of an atom to attract electrons towards this atom. The first scale of electronegativity was developed by linus pauling and on his scale copper has a value of. Copper Electronegativity.
From cursorme.blogspot.com
√ 15+ Electronegativity Chart Pauling Scale Cursome Idea Of Chart Copper Electronegativity In general, an atom’s electronegativity is affected by both its atomic number and the. 119 rows find the electronegativity values of all elements in a table, including copper (1.90). Electronegativity of copper is 1.9. Electronegativity is a chemical property that describes how well an atom. [ar] 3d 10 4s 1. Shorthand electron configuration of copper: The electronegativity of copper is:. Copper Electronegativity.
From mavink.com
Periodic Table With Electronegativity Values Copper Electronegativity 119 rows find the electronegativity values of all elements in a table, including copper (1.90). Shorthand electron configuration of copper: Have a question about using wolfram|alpha? Electronegativity is a chemical property that describes how well an atom. The first scale of electronegativity was developed by linus pauling and on his scale copper has a value of 1.90 on a scale. Copper Electronegativity.
From www.breakingatom.com
Electronegativity of the Elements Copper Electronegativity The electronegativity of copper is: Compute answers using wolfram's breakthrough technology & knowledgebase, relied on by. Electronegativity, symbol χ, is a chemical property that describes the tendency of an atom to attract electrons towards this atom. Electronegativity of copper is 1.9. Shorthand electron configuration of copper: Electronegativity is a chemical property that describes how well an atom. The first scale. Copper Electronegativity.
From pediabay.com
Periodic Table with Electronegativity (UltraHigh Resolution Image Copper Electronegativity Shorthand electron configuration of copper: Electronegativity of copper is 1.9. Electronegativity, symbol χ, is a chemical property that describes the tendency of an atom to attract electrons towards this atom. 119 rows find the electronegativity values of all elements in a table, including copper (1.90). Compute answers using wolfram's breakthrough technology & knowledgebase, relied on by. Have a question about. Copper Electronegativity.
From www.pnas.org
Electronegativity and chemical hardness of elements under pressure PNAS Copper Electronegativity [ar] 3d 10 4s 1. In general, an atom’s electronegativity is affected by both its atomic number and the. The electronegativity of copper is: Compute answers using wolfram's breakthrough technology & knowledgebase, relied on by. Shorthand electron configuration of copper: Electronegativity, symbol χ, is a chemical property that describes the tendency of an atom to attract electrons towards this atom.. Copper Electronegativity.
From anthonystrendsassignment.weebly.com
Electronegativity Periodic Trends Copper Electronegativity Electronegativity, symbol χ, is a chemical property that describes the tendency of an atom to attract electrons towards this atom. Electronegativity of copper is 1.9. [ar] 3d 10 4s 1. Compute answers using wolfram's breakthrough technology & knowledgebase, relied on by. In general, an atom’s electronegativity is affected by both its atomic number and the. Electronegativity is a chemical property. Copper Electronegativity.
From www.youtube.com
Is Cu(OH)2, Copper (II) hydroxide, Ionic or Covalent? YouTube Copper Electronegativity Electronegativity is a chemical property that describes how well an atom. Electronegativity, symbol χ, is a chemical property that describes the tendency of an atom to attract electrons towards this atom. Shorthand electron configuration of copper: [ar] 3d 10 4s 1. Have a question about using wolfram|alpha? Electronegativity of copper is 1.9. In general, an atom’s electronegativity is affected by. Copper Electronegativity.
From www.dreamstime.com
Electronegativity Periodic Table Stock Illustration Illustration of Copper Electronegativity Electronegativity of copper is 1.9. Compute answers using wolfram's breakthrough technology & knowledgebase, relied on by. 119 rows find the electronegativity values of all elements in a table, including copper (1.90). The first scale of electronegativity was developed by linus pauling and on his scale copper has a value of 1.90 on a scale running from from. [ar] 3d 10. Copper Electronegativity.
From chem.libretexts.org
8.4 Bond Polarity and Electronegativity Chemistry LibreTexts Copper Electronegativity The electronegativity of copper is: Electronegativity is a chemical property that describes how well an atom. Electronegativity of copper is 1.9. Shorthand electron configuration of copper: [ar] 3d 10 4s 1. Compute answers using wolfram's breakthrough technology & knowledgebase, relied on by. In general, an atom’s electronegativity is affected by both its atomic number and the. Electronegativity, symbol χ, is. Copper Electronegativity.
From www.collegesearch.in
Electronegativity Definitions, History, Most and Least, Impact Copper Electronegativity Have a question about using wolfram|alpha? The electronegativity of copper is: The first scale of electronegativity was developed by linus pauling and on his scale copper has a value of 1.90 on a scale running from from. Shorthand electron configuration of copper: Compute answers using wolfram's breakthrough technology & knowledgebase, relied on by. 119 rows find the electronegativity values of. Copper Electronegativity.
From www.freepik.com
Premium Vector Copper chemical element with 29 atomic number atomic Copper Electronegativity Compute answers using wolfram's breakthrough technology & knowledgebase, relied on by. The electronegativity of copper is: 119 rows find the electronegativity values of all elements in a table, including copper (1.90). In general, an atom’s electronegativity is affected by both its atomic number and the. The first scale of electronegativity was developed by linus pauling and on his scale copper. Copper Electronegativity.
From worksheetlistci.z21.web.core.windows.net
Lesson 7.5 Electronegativity And Polarity Copper Electronegativity [ar] 3d 10 4s 1. Have a question about using wolfram|alpha? Electronegativity is a chemical property that describes how well an atom. In general, an atom’s electronegativity is affected by both its atomic number and the. Electronegativity, symbol χ, is a chemical property that describes the tendency of an atom to attract electrons towards this atom. Shorthand electron configuration of. Copper Electronegativity.
From chemistry.com.pk
Electronegativity and Electronegativity Chart in PDF Copper Electronegativity Shorthand electron configuration of copper: Electronegativity, symbol χ, is a chemical property that describes the tendency of an atom to attract electrons towards this atom. Electronegativity is a chemical property that describes how well an atom. 119 rows find the electronegativity values of all elements in a table, including copper (1.90). [ar] 3d 10 4s 1. Have a question about. Copper Electronegativity.
From mmerevise.co.uk
Electronegativity & Intermolecular Forces MME Copper Electronegativity Electronegativity of copper is 1.9. 119 rows find the electronegativity values of all elements in a table, including copper (1.90). The first scale of electronegativity was developed by linus pauling and on his scale copper has a value of 1.90 on a scale running from from. The electronegativity of copper is: Compute answers using wolfram's breakthrough technology & knowledgebase, relied. Copper Electronegativity.
From pubchem.ncbi.nlm.nih.gov
Electronegativity Periodic Table of Elements PubChem Copper Electronegativity The electronegativity of copper is: Electronegativity, symbol χ, is a chemical property that describes the tendency of an atom to attract electrons towards this atom. Compute answers using wolfram's breakthrough technology & knowledgebase, relied on by. Electronegativity is a chemical property that describes how well an atom. In general, an atom’s electronegativity is affected by both its atomic number and. Copper Electronegativity.
From www.dreamstime.com
Copper Chemical Element with First Ionization Energy, Atomic Mass and Copper Electronegativity In general, an atom’s electronegativity is affected by both its atomic number and the. The first scale of electronegativity was developed by linus pauling and on his scale copper has a value of 1.90 on a scale running from from. 119 rows find the electronegativity values of all elements in a table, including copper (1.90). [ar] 3d 10 4s 1.. Copper Electronegativity.
From ar.inspiredpencil.com
Periodic Table With Electronegativity And Electron Configuration Copper Electronegativity Electronegativity is a chemical property that describes how well an atom. 119 rows find the electronegativity values of all elements in a table, including copper (1.90). In general, an atom’s electronegativity is affected by both its atomic number and the. Electronegativity of copper is 1.9. The first scale of electronegativity was developed by linus pauling and on his scale copper. Copper Electronegativity.
From www.chemistrylearner.com
Electronegativity Definition, Value Chart, and Trend in Periodic Table Copper Electronegativity The first scale of electronegativity was developed by linus pauling and on his scale copper has a value of 1.90 on a scale running from from. Electronegativity of copper is 1.9. Electronegativity is a chemical property that describes how well an atom. The electronegativity of copper is: In general, an atom’s electronegativity is affected by both its atomic number and. Copper Electronegativity.
From material-properties.org
Copper Periodic Table and Atomic Properties Copper Electronegativity 119 rows find the electronegativity values of all elements in a table, including copper (1.90). Electronegativity of copper is 1.9. Electronegativity, symbol χ, is a chemical property that describes the tendency of an atom to attract electrons towards this atom. Have a question about using wolfram|alpha? Compute answers using wolfram's breakthrough technology & knowledgebase, relied on by. The first scale. Copper Electronegativity.
From www.naturehacker.org
All I know is I know nothing. New Dielectric materials Barium Copper Copper Electronegativity Electronegativity of copper is 1.9. The first scale of electronegativity was developed by linus pauling and on his scale copper has a value of 1.90 on a scale running from from. Compute answers using wolfram's breakthrough technology & knowledgebase, relied on by. The electronegativity of copper is: Electronegativity is a chemical property that describes how well an atom. Electronegativity, symbol. Copper Electronegativity.