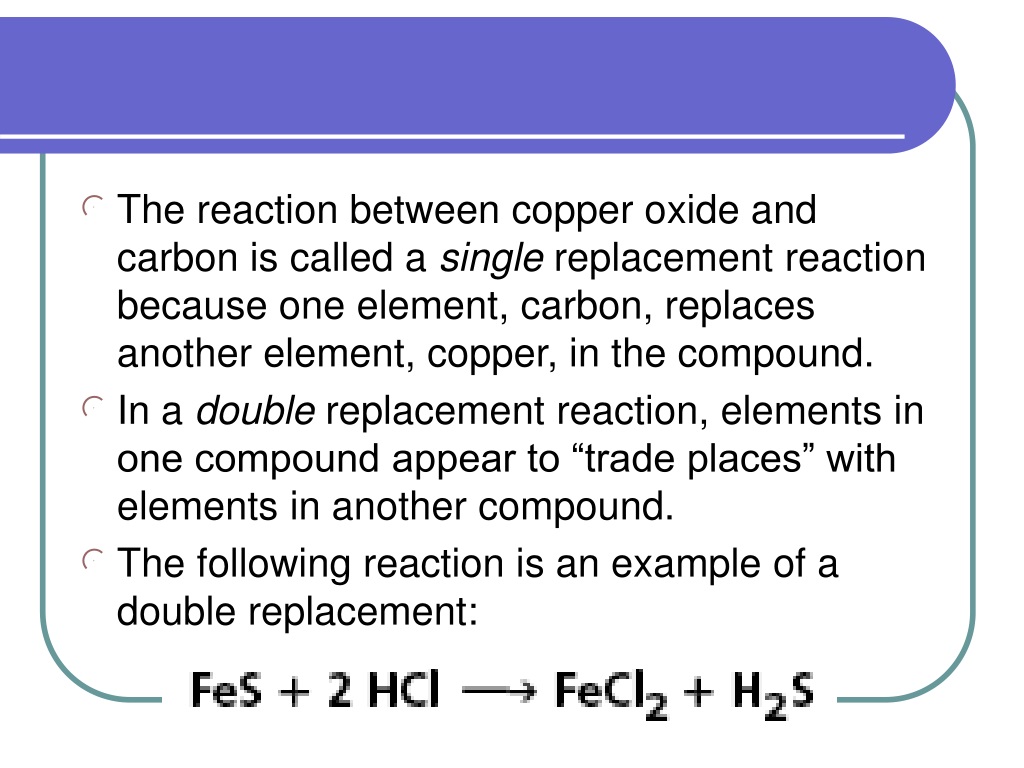
Copper Oxide And Carbon . If you heat a mixture in a test tube, a red glow runs through the mixture showing that heat. In this experiment, students heat two metal oxides with powdered charcoal. There are two parts to the problem; Carbon and copper oxide part 1 the two unnamed black solids are known to be either copper(ii) oxide or carbon. Copper oxide + carbon → copper + carbon dioxide. The crushed rock is mixed with carbon and heated strongly. Cu oxides catalyze the electrochemical carbon dioxide reduction reaction (co2rr) to hydrocarbons and oxygenates with. Copper oxide + carbon → copper + carbon dioxide Explore this two part problem and explore dilute acids and the reduction of metal oxides. Therefore, in this study, catalytic oxidation mechanisms of co over copper oxide (cuo) with interfering gases involved are. The reaction between carbon and copper(ii) oxide carbon powder and copper(ii) oxide are both black. 2cuo (s) + c (s) → 2cu (l) + co 2 (g) in this reaction, carbon is oxidised because. If the carbon is more reactive than the metal it will remove the oxygen from the metal oxide and leave. Devise three or more different.
from www.slideserve.com
Carbon and copper oxide part 1 the two unnamed black solids are known to be either copper(ii) oxide or carbon. The reaction between carbon and copper(ii) oxide carbon powder and copper(ii) oxide are both black. If the carbon is more reactive than the metal it will remove the oxygen from the metal oxide and leave. If you heat a mixture in a test tube, a red glow runs through the mixture showing that heat. There are two parts to the problem; Explore this two part problem and explore dilute acids and the reduction of metal oxides. Devise three or more different. In this experiment, students heat two metal oxides with powdered charcoal. The crushed rock is mixed with carbon and heated strongly. 2cuo (s) + c (s) → 2cu (l) + co 2 (g) in this reaction, carbon is oxidised because.
PPT Describing Chemical Reactions PowerPoint Presentation, free
Copper Oxide And Carbon Explore this two part problem and explore dilute acids and the reduction of metal oxides. If you heat a mixture in a test tube, a red glow runs through the mixture showing that heat. In this experiment, students heat two metal oxides with powdered charcoal. Copper oxide + carbon → copper + carbon dioxide There are two parts to the problem; Cu oxides catalyze the electrochemical carbon dioxide reduction reaction (co2rr) to hydrocarbons and oxygenates with. The crushed rock is mixed with carbon and heated strongly. 2cuo (s) + c (s) → 2cu (l) + co 2 (g) in this reaction, carbon is oxidised because. The reaction between carbon and copper(ii) oxide carbon powder and copper(ii) oxide are both black. If the carbon is more reactive than the metal it will remove the oxygen from the metal oxide and leave. Therefore, in this study, catalytic oxidation mechanisms of co over copper oxide (cuo) with interfering gases involved are. Explore this two part problem and explore dilute acids and the reduction of metal oxides. Copper oxide + carbon → copper + carbon dioxide. Carbon and copper oxide part 1 the two unnamed black solids are known to be either copper(ii) oxide or carbon. Devise three or more different.
From sitn.hms.harvard.edu
Recycling Carbon Dioxide with Copper Catalysts Science in the News Copper Oxide And Carbon If the carbon is more reactive than the metal it will remove the oxygen from the metal oxide and leave. The crushed rock is mixed with carbon and heated strongly. If you heat a mixture in a test tube, a red glow runs through the mixture showing that heat. Devise three or more different. There are two parts to the. Copper Oxide And Carbon.
From kneepast.telepathie.net
Here’s A Quick Way To Solve A Tips About How To Reduce Copper Oxide Copper Oxide And Carbon The reaction between carbon and copper(ii) oxide carbon powder and copper(ii) oxide are both black. If the carbon is more reactive than the metal it will remove the oxygen from the metal oxide and leave. Carbon and copper oxide part 1 the two unnamed black solids are known to be either copper(ii) oxide or carbon. Therefore, in this study, catalytic. Copper Oxide And Carbon.
From studylib.net
What mass of copper oxide can we make from copper carbonate Copper Oxide And Carbon Therefore, in this study, catalytic oxidation mechanisms of co over copper oxide (cuo) with interfering gases involved are. Devise three or more different. Carbon and copper oxide part 1 the two unnamed black solids are known to be either copper(ii) oxide or carbon. In this experiment, students heat two metal oxides with powdered charcoal. 2cuo (s) + c (s) →. Copper Oxide And Carbon.
From www.youtube.com
Carbon reduction of Copper Oxide YouTube Copper Oxide And Carbon Therefore, in this study, catalytic oxidation mechanisms of co over copper oxide (cuo) with interfering gases involved are. The crushed rock is mixed with carbon and heated strongly. There are two parts to the problem; The reaction between carbon and copper(ii) oxide carbon powder and copper(ii) oxide are both black. Carbon and copper oxide part 1 the two unnamed black. Copper Oxide And Carbon.
From www.istockphoto.com
Reduction Of Copper Oxide With Carbon Stock Photo Download Image Now Copper Oxide And Carbon Copper oxide + carbon → copper + carbon dioxide. The reaction between carbon and copper(ii) oxide carbon powder and copper(ii) oxide are both black. In this experiment, students heat two metal oxides with powdered charcoal. Carbon and copper oxide part 1 the two unnamed black solids are known to be either copper(ii) oxide or carbon. Copper oxide + carbon →. Copper Oxide And Carbon.
From loeqyugeg.blob.core.windows.net
Copper(Ii) Oxide And Carbon Equation at John Wyllie blog Copper Oxide And Carbon In this experiment, students heat two metal oxides with powdered charcoal. Copper oxide + carbon → copper + carbon dioxide The reaction between carbon and copper(ii) oxide carbon powder and copper(ii) oxide are both black. 2cuo (s) + c (s) → 2cu (l) + co 2 (g) in this reaction, carbon is oxidised because. Cu oxides catalyze the electrochemical carbon. Copper Oxide And Carbon.
From loeqyugeg.blob.core.windows.net
Copper(Ii) Oxide And Carbon Equation at John Wyllie blog Copper Oxide And Carbon Explore this two part problem and explore dilute acids and the reduction of metal oxides. Copper oxide + carbon → copper + carbon dioxide The crushed rock is mixed with carbon and heated strongly. Devise three or more different. If the carbon is more reactive than the metal it will remove the oxygen from the metal oxide and leave. In. Copper Oxide And Carbon.
From pubs.acs.org
Photocatalytic CC Coupling from Carbon Dioxide Reduction on Copper Copper Oxide And Carbon If the carbon is more reactive than the metal it will remove the oxygen from the metal oxide and leave. In this experiment, students heat two metal oxides with powdered charcoal. The crushed rock is mixed with carbon and heated strongly. Carbon and copper oxide part 1 the two unnamed black solids are known to be either copper(ii) oxide or. Copper Oxide And Carbon.
From www.slideserve.com
PPT Extracting Copper PowerPoint Presentation, free download ID4308024 Copper Oxide And Carbon The reaction between carbon and copper(ii) oxide carbon powder and copper(ii) oxide are both black. Therefore, in this study, catalytic oxidation mechanisms of co over copper oxide (cuo) with interfering gases involved are. In this experiment, students heat two metal oxides with powdered charcoal. Devise three or more different. If you heat a mixture in a test tube, a red. Copper Oxide And Carbon.
From www.bbc.co.uk
BBC GCSE Bitesize Thermal Copper Oxide And Carbon Copper oxide + carbon → copper + carbon dioxide. Devise three or more different. The reaction between carbon and copper(ii) oxide carbon powder and copper(ii) oxide are both black. Carbon and copper oxide part 1 the two unnamed black solids are known to be either copper(ii) oxide or carbon. 2cuo (s) + c (s) → 2cu (l) + co 2. Copper Oxide And Carbon.
From pubs.acs.org
Branched Copper Oxide Nanoparticles Induce Highly Selective Ethylene Copper Oxide And Carbon In this experiment, students heat two metal oxides with powdered charcoal. Cu oxides catalyze the electrochemical carbon dioxide reduction reaction (co2rr) to hydrocarbons and oxygenates with. If the carbon is more reactive than the metal it will remove the oxygen from the metal oxide and leave. Explore this two part problem and explore dilute acids and the reduction of metal. Copper Oxide And Carbon.
From www.slideshare.net
Chapter 4 Copper Oxide And Carbon If the carbon is more reactive than the metal it will remove the oxygen from the metal oxide and leave. Carbon and copper oxide part 1 the two unnamed black solids are known to be either copper(ii) oxide or carbon. The crushed rock is mixed with carbon and heated strongly. Copper oxide + carbon → copper + carbon dioxide 2cuo. Copper Oxide And Carbon.
From www.youtube.com
of Copper Carbonate YouTube Copper Oxide And Carbon If the carbon is more reactive than the metal it will remove the oxygen from the metal oxide and leave. Cu oxides catalyze the electrochemical carbon dioxide reduction reaction (co2rr) to hydrocarbons and oxygenates with. Devise three or more different. If you heat a mixture in a test tube, a red glow runs through the mixture showing that heat. The. Copper Oxide And Carbon.
From www.slideserve.com
PPT Describing Chemical Reactions PowerPoint Presentation, free Copper Oxide And Carbon Cu oxides catalyze the electrochemical carbon dioxide reduction reaction (co2rr) to hydrocarbons and oxygenates with. Copper oxide + carbon → copper + carbon dioxide The reaction between carbon and copper(ii) oxide carbon powder and copper(ii) oxide are both black. In this experiment, students heat two metal oxides with powdered charcoal. Therefore, in this study, catalytic oxidation mechanisms of co over. Copper Oxide And Carbon.
From www.researchgate.net
XPS spectrum of copper oxide/carbon synthesized by Copper Oxide And Carbon Copper oxide + carbon → copper + carbon dioxide. There are two parts to the problem; If you heat a mixture in a test tube, a red glow runs through the mixture showing that heat. 2cuo (s) + c (s) → 2cu (l) + co 2 (g) in this reaction, carbon is oxidised because. Carbon and copper oxide part 1. Copper Oxide And Carbon.
From www.alamy.com
reaction copper carbonate to copper oxide and carbon Copper Oxide And Carbon Explore this two part problem and explore dilute acids and the reduction of metal oxides. Copper oxide + carbon → copper + carbon dioxide The reaction between carbon and copper(ii) oxide carbon powder and copper(ii) oxide are both black. The crushed rock is mixed with carbon and heated strongly. Copper oxide + carbon → copper + carbon dioxide. There are. Copper Oxide And Carbon.
From www.researchgate.net
XPS spectrum of copper oxide/carbon synthesized by Copper Oxide And Carbon Explore this two part problem and explore dilute acids and the reduction of metal oxides. The crushed rock is mixed with carbon and heated strongly. 2cuo (s) + c (s) → 2cu (l) + co 2 (g) in this reaction, carbon is oxidised because. Copper oxide + carbon → copper + carbon dioxide Copper oxide + carbon → copper +. Copper Oxide And Carbon.
From exospybey.blob.core.windows.net
Copper Ii Oxide And Carbon Dioxide at Carmen Gadson blog Copper Oxide And Carbon The crushed rock is mixed with carbon and heated strongly. There are two parts to the problem; If you heat a mixture in a test tube, a red glow runs through the mixture showing that heat. Devise three or more different. If the carbon is more reactive than the metal it will remove the oxygen from the metal oxide and. Copper Oxide And Carbon.
From www.researchgate.net
Experimental diagram of copper oxide doped different carbon substrates Copper Oxide And Carbon The crushed rock is mixed with carbon and heated strongly. If the carbon is more reactive than the metal it will remove the oxygen from the metal oxide and leave. Therefore, in this study, catalytic oxidation mechanisms of co over copper oxide (cuo) with interfering gases involved are. Carbon and copper oxide part 1 the two unnamed black solids are. Copper Oxide And Carbon.
From www.alamy.com
Copper oxide reaction hires stock photography and images Alamy Copper Oxide And Carbon The reaction between carbon and copper(ii) oxide carbon powder and copper(ii) oxide are both black. Therefore, in this study, catalytic oxidation mechanisms of co over copper oxide (cuo) with interfering gases involved are. Cu oxides catalyze the electrochemical carbon dioxide reduction reaction (co2rr) to hydrocarbons and oxygenates with. There are two parts to the problem; Copper oxide + carbon →. Copper Oxide And Carbon.
From www.semanticscholar.org
Figure 2 from In situ growth of copper oxidegraphite carbon nitride Copper Oxide And Carbon Copper oxide + carbon → copper + carbon dioxide Explore this two part problem and explore dilute acids and the reduction of metal oxides. In this experiment, students heat two metal oxides with powdered charcoal. Cu oxides catalyze the electrochemical carbon dioxide reduction reaction (co2rr) to hydrocarbons and oxygenates with. Carbon and copper oxide part 1 the two unnamed black. Copper Oxide And Carbon.
From www.researchgate.net
Carbon dioxide reduction over coppercuprous oxide electrodes prepared Copper Oxide And Carbon Explore this two part problem and explore dilute acids and the reduction of metal oxides. Devise three or more different. Carbon and copper oxide part 1 the two unnamed black solids are known to be either copper(ii) oxide or carbon. The reaction between carbon and copper(ii) oxide carbon powder and copper(ii) oxide are both black. Therefore, in this study, catalytic. Copper Oxide And Carbon.
From www.toppr.com
Copper carbonate to copper oxide and carbon dioxide. Guess Copper Oxide And Carbon There are two parts to the problem; Copper oxide + carbon → copper + carbon dioxide. The crushed rock is mixed with carbon and heated strongly. Copper oxide + carbon → copper + carbon dioxide If you heat a mixture in a test tube, a red glow runs through the mixture showing that heat. 2cuo (s) + c (s) →. Copper Oxide And Carbon.
From www.youtube.com
Heating a mixture of copper (II) oxide and carbon C0118 YouTube Copper Oxide And Carbon Explore this two part problem and explore dilute acids and the reduction of metal oxides. Carbon and copper oxide part 1 the two unnamed black solids are known to be either copper(ii) oxide or carbon. 2cuo (s) + c (s) → 2cu (l) + co 2 (g) in this reaction, carbon is oxidised because. Therefore, in this study, catalytic oxidation. Copper Oxide And Carbon.
From studylib.net
Redox reaction between copper oxide and carbon Copper Oxide And Carbon Cu oxides catalyze the electrochemical carbon dioxide reduction reaction (co2rr) to hydrocarbons and oxygenates with. The reaction between carbon and copper(ii) oxide carbon powder and copper(ii) oxide are both black. Copper oxide + carbon → copper + carbon dioxide. The crushed rock is mixed with carbon and heated strongly. Therefore, in this study, catalytic oxidation mechanisms of co over copper. Copper Oxide And Carbon.
From www.slideserve.com
PPT Extracting Copper PowerPoint Presentation, free download ID4308025 Copper Oxide And Carbon Therefore, in this study, catalytic oxidation mechanisms of co over copper oxide (cuo) with interfering gases involved are. Copper oxide + carbon → copper + carbon dioxide. There are two parts to the problem; In this experiment, students heat two metal oxides with powdered charcoal. Copper oxide + carbon → copper + carbon dioxide The crushed rock is mixed with. Copper Oxide And Carbon.
From exospybey.blob.core.windows.net
Copper Ii Oxide And Carbon Dioxide at Carmen Gadson blog Copper Oxide And Carbon In this experiment, students heat two metal oxides with powdered charcoal. Copper oxide + carbon → copper + carbon dioxide The crushed rock is mixed with carbon and heated strongly. If the carbon is more reactive than the metal it will remove the oxygen from the metal oxide and leave. Copper oxide + carbon → copper + carbon dioxide. There. Copper Oxide And Carbon.
From www.mdpi.com
IJMS Free FullText Highly Selective Electrochemical CO2 Reduction Copper Oxide And Carbon Therefore, in this study, catalytic oxidation mechanisms of co over copper oxide (cuo) with interfering gases involved are. The crushed rock is mixed with carbon and heated strongly. Copper oxide + carbon → copper + carbon dioxide. Copper oxide + carbon → copper + carbon dioxide Devise three or more different. If the carbon is more reactive than the metal. Copper Oxide And Carbon.
From blog.thepipingmart.com
Copper Oxidation States and Colors Copper Oxide And Carbon 2cuo (s) + c (s) → 2cu (l) + co 2 (g) in this reaction, carbon is oxidised because. If the carbon is more reactive than the metal it will remove the oxygen from the metal oxide and leave. Copper oxide + carbon → copper + carbon dioxide Explore this two part problem and explore dilute acids and the reduction. Copper Oxide And Carbon.
From www.youtube.com
of Copper (II) Carbonate Experiment YouTube Copper Oxide And Carbon The crushed rock is mixed with carbon and heated strongly. There are two parts to the problem; Carbon and copper oxide part 1 the two unnamed black solids are known to be either copper(ii) oxide or carbon. In this experiment, students heat two metal oxides with powdered charcoal. Therefore, in this study, catalytic oxidation mechanisms of co over copper oxide. Copper Oxide And Carbon.
From www.online-sciences.com
Types of chemical reactions and Thermal reactions Copper Oxide And Carbon If the carbon is more reactive than the metal it will remove the oxygen from the metal oxide and leave. Carbon and copper oxide part 1 the two unnamed black solids are known to be either copper(ii) oxide or carbon. The reaction between carbon and copper(ii) oxide carbon powder and copper(ii) oxide are both black. In this experiment, students heat. Copper Oxide And Carbon.
From spmchemistry.blog.onlinetuition.com.my
Position of Carbon in The Series of Reactivity of Metals SPM Chemistry Copper Oxide And Carbon 2cuo (s) + c (s) → 2cu (l) + co 2 (g) in this reaction, carbon is oxidised because. Explore this two part problem and explore dilute acids and the reduction of metal oxides. If the carbon is more reactive than the metal it will remove the oxygen from the metal oxide and leave. In this experiment, students heat two. Copper Oxide And Carbon.
From loeqyugeg.blob.core.windows.net
Copper(Ii) Oxide And Carbon Equation at John Wyllie blog Copper Oxide And Carbon Carbon and copper oxide part 1 the two unnamed black solids are known to be either copper(ii) oxide or carbon. Therefore, in this study, catalytic oxidation mechanisms of co over copper oxide (cuo) with interfering gases involved are. Copper oxide + carbon → copper + carbon dioxide The reaction between carbon and copper(ii) oxide carbon powder and copper(ii) oxide are. Copper Oxide And Carbon.
From www.youtube.com
Heating a mixture of carbon and copper (II) oxide YouTube Copper Oxide And Carbon The reaction between carbon and copper(ii) oxide carbon powder and copper(ii) oxide are both black. Copper oxide + carbon → copper + carbon dioxide. 2cuo (s) + c (s) → 2cu (l) + co 2 (g) in this reaction, carbon is oxidised because. There are two parts to the problem; Cu oxides catalyze the electrochemical carbon dioxide reduction reaction (co2rr). Copper Oxide And Carbon.
From brainly.com
Water gas 3 Copper oxide and carbon dioxide are made when copper Copper Oxide And Carbon 2cuo (s) + c (s) → 2cu (l) + co 2 (g) in this reaction, carbon is oxidised because. Copper oxide + carbon → copper + carbon dioxide Carbon and copper oxide part 1 the two unnamed black solids are known to be either copper(ii) oxide or carbon. If you heat a mixture in a test tube, a red glow. Copper Oxide And Carbon.