Ir Spectroscopy Bond Strength . Infrared (ir) spectroscopy can be used to determine functional groups and bond strengths based upon molecular vibrations. Infrared spectroscopy is based on molecular vibrations caused by the oscillation. Absorption of infrared radiation brings about changes in molecular vibrations within molecules and ‘measurements’ of the ways in which bonds vibrate gives rise. Visualizing different vibrational energy levels. It follows from all the results that the data on the stretching frequencies of different bonds may be regarded as a source of the. The strength of the bond or bond order also has an impact on frequency. The “ball and spring” model for bond vibrations. Using the force constant k (which reflects the stiffness of the spring) and the two masses m1 and m2, then the equation indicates how the frequency, u, of the absorption should change.
from studylib.net
Absorption of infrared radiation brings about changes in molecular vibrations within molecules and ‘measurements’ of the ways in which bonds vibrate gives rise. The “ball and spring” model for bond vibrations. The strength of the bond or bond order also has an impact on frequency. Visualizing different vibrational energy levels. Infrared spectroscopy is based on molecular vibrations caused by the oscillation. Using the force constant k (which reflects the stiffness of the spring) and the two masses m1 and m2, then the equation indicates how the frequency, u, of the absorption should change. It follows from all the results that the data on the stretching frequencies of different bonds may be regarded as a source of the. Infrared (ir) spectroscopy can be used to determine functional groups and bond strengths based upon molecular vibrations.
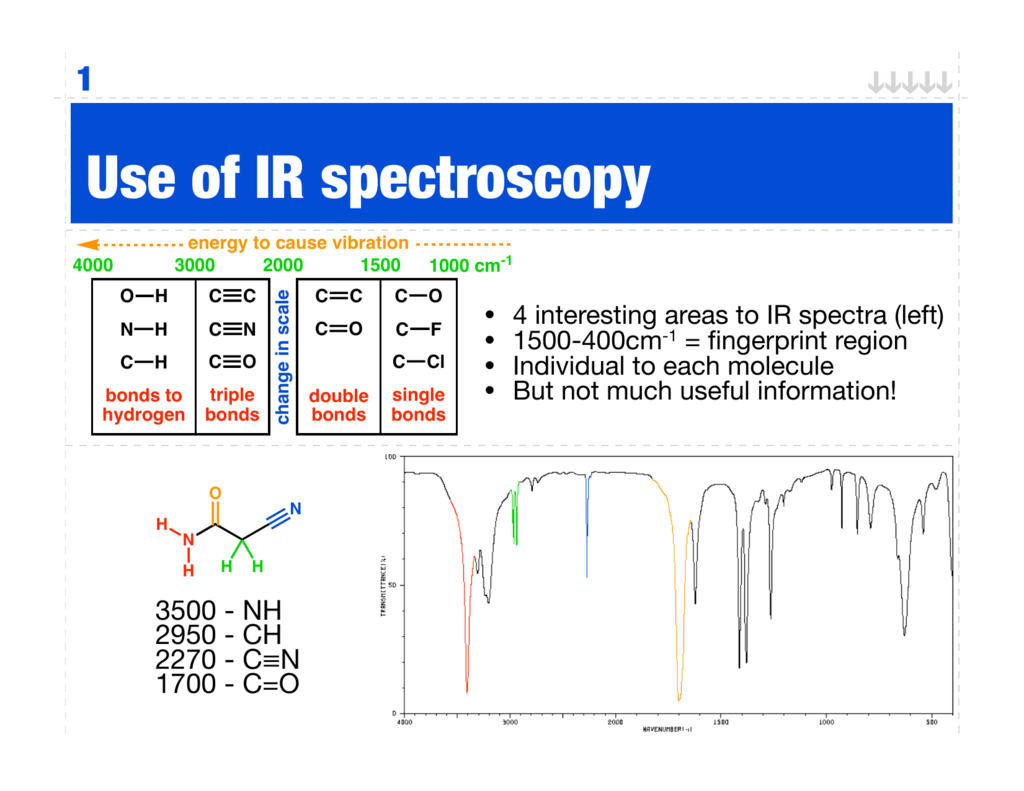
Use of IR spectroscopy
Ir Spectroscopy Bond Strength The strength of the bond or bond order also has an impact on frequency. Using the force constant k (which reflects the stiffness of the spring) and the two masses m1 and m2, then the equation indicates how the frequency, u, of the absorption should change. It follows from all the results that the data on the stretching frequencies of different bonds may be regarded as a source of the. Visualizing different vibrational energy levels. Infrared (ir) spectroscopy can be used to determine functional groups and bond strengths based upon molecular vibrations. The strength of the bond or bond order also has an impact on frequency. The “ball and spring” model for bond vibrations. Infrared spectroscopy is based on molecular vibrations caused by the oscillation. Absorption of infrared radiation brings about changes in molecular vibrations within molecules and ‘measurements’ of the ways in which bonds vibrate gives rise.
From www.researchgate.net
FTIR spectrum of NH and C=O stretching regions of linear and branched Ir Spectroscopy Bond Strength Using the force constant k (which reflects the stiffness of the spring) and the two masses m1 and m2, then the equation indicates how the frequency, u, of the absorption should change. The strength of the bond or bond order also has an impact on frequency. Absorption of infrared radiation brings about changes in molecular vibrations within molecules and ‘measurements’. Ir Spectroscopy Bond Strength.
From www.slideserve.com
PPT Infrared Spectroscopy Theory and Interpretation of IR spectra Ir Spectroscopy Bond Strength Using the force constant k (which reflects the stiffness of the spring) and the two masses m1 and m2, then the equation indicates how the frequency, u, of the absorption should change. It follows from all the results that the data on the stretching frequencies of different bonds may be regarded as a source of the. Infrared (ir) spectroscopy can. Ir Spectroscopy Bond Strength.
From www.youtube.com
Introduction to IR Spectroscopy How to Read an Infrared Spectroscopy Ir Spectroscopy Bond Strength It follows from all the results that the data on the stretching frequencies of different bonds may be regarded as a source of the. The “ball and spring” model for bond vibrations. Using the force constant k (which reflects the stiffness of the spring) and the two masses m1 and m2, then the equation indicates how the frequency, u, of. Ir Spectroscopy Bond Strength.
From www.researchgate.net
1. The correlation table represents infrared absorption positions and Ir Spectroscopy Bond Strength The “ball and spring” model for bond vibrations. Infrared (ir) spectroscopy can be used to determine functional groups and bond strengths based upon molecular vibrations. Visualizing different vibrational energy levels. Infrared spectroscopy is based on molecular vibrations caused by the oscillation. Using the force constant k (which reflects the stiffness of the spring) and the two masses m1 and m2,. Ir Spectroscopy Bond Strength.
From dxomntmti.blob.core.windows.net
Infrared Spectroscopy Functional Groups Table at Carla Brown blog Ir Spectroscopy Bond Strength The “ball and spring” model for bond vibrations. It follows from all the results that the data on the stretching frequencies of different bonds may be regarded as a source of the. Absorption of infrared radiation brings about changes in molecular vibrations within molecules and ‘measurements’ of the ways in which bonds vibrate gives rise. Infrared (ir) spectroscopy can be. Ir Spectroscopy Bond Strength.
From www.researchgate.net
Fouriertransform infrared spectroscopy (FTIR) curves between (a) 4000 Ir Spectroscopy Bond Strength The “ball and spring” model for bond vibrations. The strength of the bond or bond order also has an impact on frequency. Absorption of infrared radiation brings about changes in molecular vibrations within molecules and ‘measurements’ of the ways in which bonds vibrate gives rise. Visualizing different vibrational energy levels. Using the force constant k (which reflects the stiffness of. Ir Spectroscopy Bond Strength.
From www.animalia-life.club
Ir Spectrum Table Functional Groups Ir Spectroscopy Bond Strength The “ball and spring” model for bond vibrations. Absorption of infrared radiation brings about changes in molecular vibrations within molecules and ‘measurements’ of the ways in which bonds vibrate gives rise. Infrared spectroscopy is based on molecular vibrations caused by the oscillation. Using the force constant k (which reflects the stiffness of the spring) and the two masses m1 and. Ir Spectroscopy Bond Strength.
From chem.libretexts.org
3.7 Interpreting Infrared Spectra Chemistry LibreTexts Ir Spectroscopy Bond Strength The strength of the bond or bond order also has an impact on frequency. It follows from all the results that the data on the stretching frequencies of different bonds may be regarded as a source of the. The “ball and spring” model for bond vibrations. Visualizing different vibrational energy levels. Infrared (ir) spectroscopy can be used to determine functional. Ir Spectroscopy Bond Strength.
From www.youtube.com
IR Spectroscopy Basic Introduction YouTube Ir Spectroscopy Bond Strength The “ball and spring” model for bond vibrations. Visualizing different vibrational energy levels. Infrared spectroscopy is based on molecular vibrations caused by the oscillation. Absorption of infrared radiation brings about changes in molecular vibrations within molecules and ‘measurements’ of the ways in which bonds vibrate gives rise. The strength of the bond or bond order also has an impact on. Ir Spectroscopy Bond Strength.
From organicchemistoncall.com
Most Commonly Used IR Spectroscopy Values In Organic Chemistry The Ir Spectroscopy Bond Strength Using the force constant k (which reflects the stiffness of the spring) and the two masses m1 and m2, then the equation indicates how the frequency, u, of the absorption should change. It follows from all the results that the data on the stretching frequencies of different bonds may be regarded as a source of the. The strength of the. Ir Spectroscopy Bond Strength.
From www.slideshare.net
Lecture10 123.101 Ir Spectroscopy Bond Strength Infrared spectroscopy is based on molecular vibrations caused by the oscillation. The strength of the bond or bond order also has an impact on frequency. Infrared (ir) spectroscopy can be used to determine functional groups and bond strengths based upon molecular vibrations. It follows from all the results that the data on the stretching frequencies of different bonds may be. Ir Spectroscopy Bond Strength.
From chemistnotes.com
How to read IR spectra 7 easy steps Chemistry Notes Ir Spectroscopy Bond Strength The “ball and spring” model for bond vibrations. Infrared spectroscopy is based on molecular vibrations caused by the oscillation. It follows from all the results that the data on the stretching frequencies of different bonds may be regarded as a source of the. Visualizing different vibrational energy levels. Absorption of infrared radiation brings about changes in molecular vibrations within molecules. Ir Spectroscopy Bond Strength.
From www.chemistrystudent.com
IR (Infrared Spectroscopy) (ALevel) ChemistryStudent Ir Spectroscopy Bond Strength It follows from all the results that the data on the stretching frequencies of different bonds may be regarded as a source of the. The strength of the bond or bond order also has an impact on frequency. Visualizing different vibrational energy levels. Infrared (ir) spectroscopy can be used to determine functional groups and bond strengths based upon molecular vibrations.. Ir Spectroscopy Bond Strength.
From www.technologynetworks.com
IR Spectroscopy and FTIR Spectroscopy How an FTIR Spectrometer Works Ir Spectroscopy Bond Strength Using the force constant k (which reflects the stiffness of the spring) and the two masses m1 and m2, then the equation indicates how the frequency, u, of the absorption should change. The strength of the bond or bond order also has an impact on frequency. The “ball and spring” model for bond vibrations. It follows from all the results. Ir Spectroscopy Bond Strength.
From www.chemistrystudent.com
IR (Infrared Spectroscopy) (ALevel) ChemistryStudent Ir Spectroscopy Bond Strength The “ball and spring” model for bond vibrations. Using the force constant k (which reflects the stiffness of the spring) and the two masses m1 and m2, then the equation indicates how the frequency, u, of the absorption should change. The strength of the bond or bond order also has an impact on frequency. Infrared (ir) spectroscopy can be used. Ir Spectroscopy Bond Strength.
From www.researchgate.net
The Fourier Transform Infrared spectroscopy spectra of the cellulose Ir Spectroscopy Bond Strength Visualizing different vibrational energy levels. Infrared (ir) spectroscopy can be used to determine functional groups and bond strengths based upon molecular vibrations. It follows from all the results that the data on the stretching frequencies of different bonds may be regarded as a source of the. The strength of the bond or bond order also has an impact on frequency.. Ir Spectroscopy Bond Strength.
From www.researchgate.net
IR spectra of 1 and 2 (a) [Ni(H2O)2(py)4][BPh4]2·4py (1); (b Ir Spectroscopy Bond Strength Visualizing different vibrational energy levels. The “ball and spring” model for bond vibrations. Infrared (ir) spectroscopy can be used to determine functional groups and bond strengths based upon molecular vibrations. The strength of the bond or bond order also has an impact on frequency. Using the force constant k (which reflects the stiffness of the spring) and the two masses. Ir Spectroscopy Bond Strength.
From www.slideserve.com
PPT Infrared Spectroscopy Theory and Interpretation of IR spectra Ir Spectroscopy Bond Strength Infrared (ir) spectroscopy can be used to determine functional groups and bond strengths based upon molecular vibrations. The “ball and spring” model for bond vibrations. The strength of the bond or bond order also has an impact on frequency. It follows from all the results that the data on the stretching frequencies of different bonds may be regarded as a. Ir Spectroscopy Bond Strength.
From awesomehome.co
Ir Spectroscopy Table Of Peaks Awesome Home Ir Spectroscopy Bond Strength The “ball and spring” model for bond vibrations. Absorption of infrared radiation brings about changes in molecular vibrations within molecules and ‘measurements’ of the ways in which bonds vibrate gives rise. Visualizing different vibrational energy levels. Infrared spectroscopy is based on molecular vibrations caused by the oscillation. Using the force constant k (which reflects the stiffness of the spring) and. Ir Spectroscopy Bond Strength.
From www.researchgate.net
The basis of infrared (IR) vibrational absorbance spectroscopy. a. The Ir Spectroscopy Bond Strength The “ball and spring” model for bond vibrations. Visualizing different vibrational energy levels. Infrared (ir) spectroscopy can be used to determine functional groups and bond strengths based upon molecular vibrations. The strength of the bond or bond order also has an impact on frequency. Infrared spectroscopy is based on molecular vibrations caused by the oscillation. Absorption of infrared radiation brings. Ir Spectroscopy Bond Strength.
From edu.rsc.org
Infrared (IR) spectroscopy Resource RSC Education Ir Spectroscopy Bond Strength The strength of the bond or bond order also has an impact on frequency. Absorption of infrared radiation brings about changes in molecular vibrations within molecules and ‘measurements’ of the ways in which bonds vibrate gives rise. The “ball and spring” model for bond vibrations. Using the force constant k (which reflects the stiffness of the spring) and the two. Ir Spectroscopy Bond Strength.
From www.masterorganicchemistry.com
Interpreting IR Specta A Quick Guide Master Organic Chemistry Ir Spectroscopy Bond Strength Using the force constant k (which reflects the stiffness of the spring) and the two masses m1 and m2, then the equation indicates how the frequency, u, of the absorption should change. The strength of the bond or bond order also has an impact on frequency. Infrared (ir) spectroscopy can be used to determine functional groups and bond strengths based. Ir Spectroscopy Bond Strength.
From www.pinterest.com
IR Spectroscopy Chart 1 Organic chemistry, Organic chemistry study Ir Spectroscopy Bond Strength The “ball and spring” model for bond vibrations. Infrared (ir) spectroscopy can be used to determine functional groups and bond strengths based upon molecular vibrations. The strength of the bond or bond order also has an impact on frequency. It follows from all the results that the data on the stretching frequencies of different bonds may be regarded as a. Ir Spectroscopy Bond Strength.
From www.animalia-life.club
Ir Spectrum Table Functional Groups Ir Spectroscopy Bond Strength The strength of the bond or bond order also has an impact on frequency. Absorption of infrared radiation brings about changes in molecular vibrations within molecules and ‘measurements’ of the ways in which bonds vibrate gives rise. The “ball and spring” model for bond vibrations. Infrared spectroscopy is based on molecular vibrations caused by the oscillation. Using the force constant. Ir Spectroscopy Bond Strength.
From studylib.net
Use of IR spectroscopy Ir Spectroscopy Bond Strength Infrared spectroscopy is based on molecular vibrations caused by the oscillation. Absorption of infrared radiation brings about changes in molecular vibrations within molecules and ‘measurements’ of the ways in which bonds vibrate gives rise. The strength of the bond or bond order also has an impact on frequency. Using the force constant k (which reflects the stiffness of the spring). Ir Spectroscopy Bond Strength.
From www.slideserve.com
PPT Hooke’s Law describes the relationship of frequency to mass and Ir Spectroscopy Bond Strength Using the force constant k (which reflects the stiffness of the spring) and the two masses m1 and m2, then the equation indicates how the frequency, u, of the absorption should change. Infrared spectroscopy is based on molecular vibrations caused by the oscillation. Infrared (ir) spectroscopy can be used to determine functional groups and bond strengths based upon molecular vibrations.. Ir Spectroscopy Bond Strength.
From www.pinterest.com
Bond Lengths and Bond Strengths for (sp3, sp2, sp) CH Bonds Bond Ir Spectroscopy Bond Strength Using the force constant k (which reflects the stiffness of the spring) and the two masses m1 and m2, then the equation indicates how the frequency, u, of the absorption should change. Visualizing different vibrational energy levels. Infrared (ir) spectroscopy can be used to determine functional groups and bond strengths based upon molecular vibrations. Absorption of infrared radiation brings about. Ir Spectroscopy Bond Strength.
From chemistrytalk.org
IR Spectroscopy ChemTalk Ir Spectroscopy Bond Strength Absorption of infrared radiation brings about changes in molecular vibrations within molecules and ‘measurements’ of the ways in which bonds vibrate gives rise. The “ball and spring” model for bond vibrations. The strength of the bond or bond order also has an impact on frequency. Infrared spectroscopy is based on molecular vibrations caused by the oscillation. It follows from all. Ir Spectroscopy Bond Strength.
From www.masterorganicchemistry.com
Bond Vibrations, Infrared Spectroscopy, and the "Ball and Spring" Model Ir Spectroscopy Bond Strength Visualizing different vibrational energy levels. The strength of the bond or bond order also has an impact on frequency. Infrared spectroscopy is based on molecular vibrations caused by the oscillation. It follows from all the results that the data on the stretching frequencies of different bonds may be regarded as a source of the. Absorption of infrared radiation brings about. Ir Spectroscopy Bond Strength.
From www.bionity.com
Infrared (IR) Spectroscopy Ir Spectroscopy Bond Strength The strength of the bond or bond order also has an impact on frequency. Infrared spectroscopy is based on molecular vibrations caused by the oscillation. Infrared (ir) spectroscopy can be used to determine functional groups and bond strengths based upon molecular vibrations. Using the force constant k (which reflects the stiffness of the spring) and the two masses m1 and. Ir Spectroscopy Bond Strength.
From www.masterorganicchemistry.com
Bond Vibrations, Infrared Spectroscopy, and the "Ball and Spring" Model Ir Spectroscopy Bond Strength Infrared (ir) spectroscopy can be used to determine functional groups and bond strengths based upon molecular vibrations. The “ball and spring” model for bond vibrations. It follows from all the results that the data on the stretching frequencies of different bonds may be regarded as a source of the. Infrared spectroscopy is based on molecular vibrations caused by the oscillation.. Ir Spectroscopy Bond Strength.
From edu.rsc.org
Infrared (IR) spectroscopy More complicated molecules Resource RSC Ir Spectroscopy Bond Strength Infrared spectroscopy is based on molecular vibrations caused by the oscillation. Visualizing different vibrational energy levels. The strength of the bond or bond order also has an impact on frequency. It follows from all the results that the data on the stretching frequencies of different bonds may be regarded as a source of the. Using the force constant k (which. Ir Spectroscopy Bond Strength.
From psiberg.com
Infrared Spectroscopy Principle, Instrumentation & Applications Ir Spectroscopy Bond Strength The “ball and spring” model for bond vibrations. Infrared (ir) spectroscopy can be used to determine functional groups and bond strengths based upon molecular vibrations. Visualizing different vibrational energy levels. It follows from all the results that the data on the stretching frequencies of different bonds may be regarded as a source of the. Absorption of infrared radiation brings about. Ir Spectroscopy Bond Strength.
From chehesj.blogspot.com
organic chemistry IR spectra and hydrogen bonds Ir Spectroscopy Bond Strength Visualizing different vibrational energy levels. The “ball and spring” model for bond vibrations. Using the force constant k (which reflects the stiffness of the spring) and the two masses m1 and m2, then the equation indicates how the frequency, u, of the absorption should change. Infrared spectroscopy is based on molecular vibrations caused by the oscillation. It follows from all. Ir Spectroscopy Bond Strength.
From www.mdpi.com
Molecules Free FullText NMR and IR Investigations of Strong Ir Spectroscopy Bond Strength Infrared spectroscopy is based on molecular vibrations caused by the oscillation. The strength of the bond or bond order also has an impact on frequency. Visualizing different vibrational energy levels. Using the force constant k (which reflects the stiffness of the spring) and the two masses m1 and m2, then the equation indicates how the frequency, u, of the absorption. Ir Spectroscopy Bond Strength.