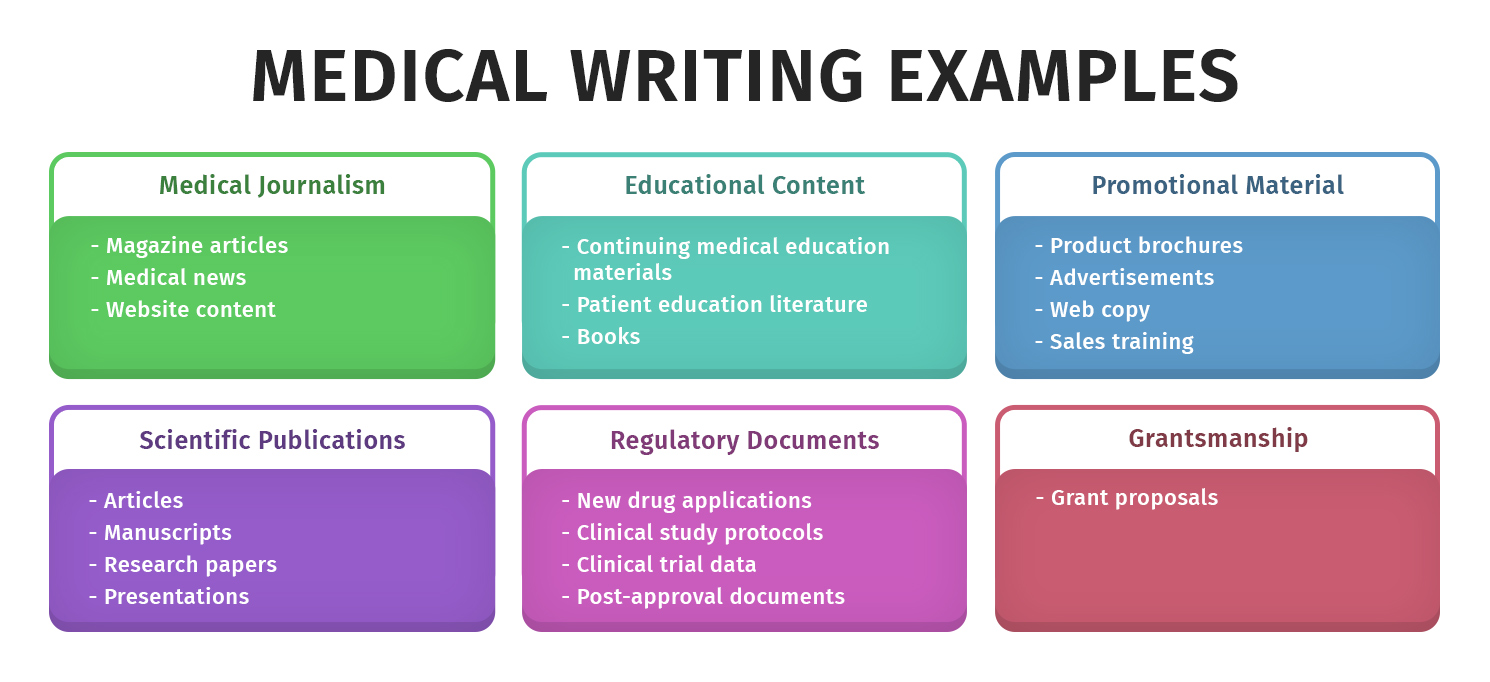
What Is Regulatory Medical Writing . What is regulatory medical writing? Medical writing is a quarterly publication that aims to educate. In the dynamic landscape of the healthcare industry, where innovation is constant and regulations are stringent, regulatory medical writing stands as a. Did minor flaws in a new drug reveal major flaws in company publication practices? Regulatory writing generally covers the gamut of documents that are involved in the drug or medical device development process, ranging from clinical trial level documents (protocols,. Regulatory medical writing means creating the documentation that regulatory agencies require in the approval process for drugs, devices and. When it comes to writing in the medical field, there are two distinct styles: Regulatory medical writing is a specialized domain within the broader field of medical writing, focusing on creating documentation. Regulatory medical writing serves as a bridge between scientific knowledge and regulatory requirements. It involves the creation of various. Regulatory writing is primarily concerned with addressing the needs of regulatory agencies and reviewers who are tasked with determining whether a drug or medical device meets the.
from technicalwriterhq.com
Regulatory medical writing serves as a bridge between scientific knowledge and regulatory requirements. Regulatory writing is primarily concerned with addressing the needs of regulatory agencies and reviewers who are tasked with determining whether a drug or medical device meets the. What is regulatory medical writing? Regulatory writing generally covers the gamut of documents that are involved in the drug or medical device development process, ranging from clinical trial level documents (protocols,. It involves the creation of various. When it comes to writing in the medical field, there are two distinct styles: Medical writing is a quarterly publication that aims to educate. Regulatory medical writing is a specialized domain within the broader field of medical writing, focusing on creating documentation. In the dynamic landscape of the healthcare industry, where innovation is constant and regulations are stringent, regulatory medical writing stands as a. Regulatory medical writing means creating the documentation that regulatory agencies require in the approval process for drugs, devices and.
10 Best Medical Writing Examples to Inspire You Technical Writer HQ
What Is Regulatory Medical Writing Regulatory writing is primarily concerned with addressing the needs of regulatory agencies and reviewers who are tasked with determining whether a drug or medical device meets the. Regulatory medical writing serves as a bridge between scientific knowledge and regulatory requirements. It involves the creation of various. Regulatory writing is primarily concerned with addressing the needs of regulatory agencies and reviewers who are tasked with determining whether a drug or medical device meets the. In the dynamic landscape of the healthcare industry, where innovation is constant and regulations are stringent, regulatory medical writing stands as a. When it comes to writing in the medical field, there are two distinct styles: Did minor flaws in a new drug reveal major flaws in company publication practices? Medical writing is a quarterly publication that aims to educate. Regulatory medical writing means creating the documentation that regulatory agencies require in the approval process for drugs, devices and. Regulatory medical writing is a specialized domain within the broader field of medical writing, focusing on creating documentation. Regulatory writing generally covers the gamut of documents that are involved in the drug or medical device development process, ranging from clinical trial level documents (protocols,. What is regulatory medical writing?
From www.slideshare.net
Careers in Medical Writing What Is Regulatory Medical Writing Regulatory medical writing is a specialized domain within the broader field of medical writing, focusing on creating documentation. It involves the creation of various. Regulatory medical writing serves as a bridge between scientific knowledge and regulatory requirements. Regulatory writing generally covers the gamut of documents that are involved in the drug or medical device development process, ranging from clinical trial. What Is Regulatory Medical Writing.
From issuu.com
Regulatory Medical Writing The Challenges of Writing Patient What Is Regulatory Medical Writing In the dynamic landscape of the healthcare industry, where innovation is constant and regulations are stringent, regulatory medical writing stands as a. Regulatory writing is primarily concerned with addressing the needs of regulatory agencies and reviewers who are tasked with determining whether a drug or medical device meets the. Did minor flaws in a new drug reveal major flaws in. What Is Regulatory Medical Writing.
From www.slideserve.com
PPT AUTOMATION IN REGULATORY MEDICAL WRITING PowerPoint Presentation What Is Regulatory Medical Writing Regulatory medical writing means creating the documentation that regulatory agencies require in the approval process for drugs, devices and. Medical writing is a quarterly publication that aims to educate. Regulatory writing is primarily concerned with addressing the needs of regulatory agencies and reviewers who are tasked with determining whether a drug or medical device meets the. When it comes to. What Is Regulatory Medical Writing.
From info.amwa.org
Medical Communicators’ Guide to Regulatory Writing What Is Regulatory Medical Writing It involves the creation of various. Did minor flaws in a new drug reveal major flaws in company publication practices? Regulatory medical writing means creating the documentation that regulatory agencies require in the approval process for drugs, devices and. What is regulatory medical writing? In the dynamic landscape of the healthcare industry, where innovation is constant and regulations are stringent,. What Is Regulatory Medical Writing.
From www.youtube.com
Regulatory Medical writing basics YouTube What Is Regulatory Medical Writing Regulatory writing generally covers the gamut of documents that are involved in the drug or medical device development process, ranging from clinical trial level documents (protocols,. Did minor flaws in a new drug reveal major flaws in company publication practices? Regulatory writing is primarily concerned with addressing the needs of regulatory agencies and reviewers who are tasked with determining whether. What Is Regulatory Medical Writing.
From formiventos.com
REGULATORY MEDICAL WRITING Formiventos What Is Regulatory Medical Writing Did minor flaws in a new drug reveal major flaws in company publication practices? Regulatory medical writing is a specialized domain within the broader field of medical writing, focusing on creating documentation. Regulatory writing is primarily concerned with addressing the needs of regulatory agencies and reviewers who are tasked with determining whether a drug or medical device meets the. Regulatory. What Is Regulatory Medical Writing.
From www.ostenhealthcare.com
Academic & Regulatory Medical Writing Training Course What Is Regulatory Medical Writing Regulatory writing is primarily concerned with addressing the needs of regulatory agencies and reviewers who are tasked with determining whether a drug or medical device meets the. What is regulatory medical writing? It involves the creation of various. Regulatory medical writing is a specialized domain within the broader field of medical writing, focusing on creating documentation. Regulatory medical writing serves. What Is Regulatory Medical Writing.
From www.slideserve.com
PPT AUTOMATION IN REGULATORY MEDICAL WRITING PowerPoint Presentation What Is Regulatory Medical Writing Medical writing is a quarterly publication that aims to educate. Regulatory medical writing means creating the documentation that regulatory agencies require in the approval process for drugs, devices and. Did minor flaws in a new drug reveal major flaws in company publication practices? Regulatory writing generally covers the gamut of documents that are involved in the drug or medical device. What Is Regulatory Medical Writing.
From www.researchgate.net
Medical writing services overview. Download Scientific Diagram What Is Regulatory Medical Writing Regulatory medical writing serves as a bridge between scientific knowledge and regulatory requirements. Regulatory writing is primarily concerned with addressing the needs of regulatory agencies and reviewers who are tasked with determining whether a drug or medical device meets the. What is regulatory medical writing? Regulatory medical writing means creating the documentation that regulatory agencies require in the approval process. What Is Regulatory Medical Writing.
From www.flickr.com
Regulatory Medical Writing Regulatory Medical Writing How… Flickr What Is Regulatory Medical Writing Medical writing is a quarterly publication that aims to educate. Regulatory medical writing serves as a bridge between scientific knowledge and regulatory requirements. When it comes to writing in the medical field, there are two distinct styles: Regulatory medical writing is a specialized domain within the broader field of medical writing, focusing on creating documentation. Regulatory writing is primarily concerned. What Is Regulatory Medical Writing.
From cromospharma.com
Regulatory & Publication medical writing Cromos Pharma What Is Regulatory Medical Writing Regulatory writing is primarily concerned with addressing the needs of regulatory agencies and reviewers who are tasked with determining whether a drug or medical device meets the. In the dynamic landscape of the healthcare industry, where innovation is constant and regulations are stringent, regulatory medical writing stands as a. Regulatory writing generally covers the gamut of documents that are involved. What Is Regulatory Medical Writing.
From www.linkedin.com
Essential Clinical and Regulatory Medical Writing Documents What Is Regulatory Medical Writing Regulatory medical writing means creating the documentation that regulatory agencies require in the approval process for drugs, devices and. Regulatory medical writing serves as a bridge between scientific knowledge and regulatory requirements. When it comes to writing in the medical field, there are two distinct styles: Did minor flaws in a new drug reveal major flaws in company publication practices?. What Is Regulatory Medical Writing.
From www.jli.edu.in
Regulatory Medical Writer James Lind Blog JLI Blog What Is Regulatory Medical Writing Regulatory medical writing serves as a bridge between scientific knowledge and regulatory requirements. It involves the creation of various. What is regulatory medical writing? Regulatory writing generally covers the gamut of documents that are involved in the drug or medical device development process, ranging from clinical trial level documents (protocols,. When it comes to writing in the medical field, there. What Is Regulatory Medical Writing.
From issuu.com
Medical writing for regulatory submission in clinical research and its What Is Regulatory Medical Writing Did minor flaws in a new drug reveal major flaws in company publication practices? Regulatory medical writing is a specialized domain within the broader field of medical writing, focusing on creating documentation. Regulatory medical writing serves as a bridge between scientific knowledge and regulatory requirements. Regulatory writing is primarily concerned with addressing the needs of regulatory agencies and reviewers who. What Is Regulatory Medical Writing.
From www.regfile.se
What about some regulatory medical writing RegFile.se What Is Regulatory Medical Writing Did minor flaws in a new drug reveal major flaws in company publication practices? Regulatory medical writing is a specialized domain within the broader field of medical writing, focusing on creating documentation. When it comes to writing in the medical field, there are two distinct styles: What is regulatory medical writing? It involves the creation of various. Regulatory writing generally. What Is Regulatory Medical Writing.
From goit.science
Regulatory Medical Writing What, why and how? Go IT What Is Regulatory Medical Writing What is regulatory medical writing? Regulatory medical writing means creating the documentation that regulatory agencies require in the approval process for drugs, devices and. When it comes to writing in the medical field, there are two distinct styles: Did minor flaws in a new drug reveal major flaws in company publication practices? Regulatory medical writing is a specialized domain within. What Is Regulatory Medical Writing.
From smiqgen.com
Regulatory Medical Writing Services Company Clinical Report Writing What Is Regulatory Medical Writing Did minor flaws in a new drug reveal major flaws in company publication practices? What is regulatory medical writing? Medical writing is a quarterly publication that aims to educate. It involves the creation of various. In the dynamic landscape of the healthcare industry, where innovation is constant and regulations are stringent, regulatory medical writing stands as a. Regulatory medical writing. What Is Regulatory Medical Writing.
From in.pinterest.com
Regulatory Medical Writing Infographic Medical, Regulatory, Writing What Is Regulatory Medical Writing Regulatory medical writing is a specialized domain within the broader field of medical writing, focusing on creating documentation. When it comes to writing in the medical field, there are two distinct styles: It involves the creation of various. In the dynamic landscape of the healthcare industry, where innovation is constant and regulations are stringent, regulatory medical writing stands as a.. What Is Regulatory Medical Writing.
From www.slideserve.com
PPT WRITING FOR MEDICAL DEVICE COMPANIES THE ROLE OF REGULATORY What Is Regulatory Medical Writing What is regulatory medical writing? Did minor flaws in a new drug reveal major flaws in company publication practices? In the dynamic landscape of the healthcare industry, where innovation is constant and regulations are stringent, regulatory medical writing stands as a. Regulatory medical writing is a specialized domain within the broader field of medical writing, focusing on creating documentation. Medical. What Is Regulatory Medical Writing.
From journal.emwa.org
Medical Writing Regulatory Writing Basics A guide to preapproval What Is Regulatory Medical Writing What is regulatory medical writing? Regulatory writing is primarily concerned with addressing the needs of regulatory agencies and reviewers who are tasked with determining whether a drug or medical device meets the. Medical writing is a quarterly publication that aims to educate. Regulatory medical writing means creating the documentation that regulatory agencies require in the approval process for drugs, devices. What Is Regulatory Medical Writing.
From especiales.europasur.es
Regulatory Medical Writing Companies. Regulatory & Medical Writing Services What Is Regulatory Medical Writing Did minor flaws in a new drug reveal major flaws in company publication practices? It involves the creation of various. In the dynamic landscape of the healthcare industry, where innovation is constant and regulations are stringent, regulatory medical writing stands as a. Regulatory medical writing serves as a bridge between scientific knowledge and regulatory requirements. Regulatory medical writing is a. What Is Regulatory Medical Writing.
From technicalwriterhq.com
10 Best Medical Writing Examples to Inspire You Technical Writer HQ What Is Regulatory Medical Writing Regulatory medical writing serves as a bridge between scientific knowledge and regulatory requirements. Did minor flaws in a new drug reveal major flaws in company publication practices? Regulatory writing is primarily concerned with addressing the needs of regulatory agencies and reviewers who are tasked with determining whether a drug or medical device meets the. What is regulatory medical writing? It. What Is Regulatory Medical Writing.
From www.slideshare.net
Sample essay on regulatory compliance What Is Regulatory Medical Writing What is regulatory medical writing? When it comes to writing in the medical field, there are two distinct styles: Regulatory medical writing means creating the documentation that regulatory agencies require in the approval process for drugs, devices and. Regulatory medical writing serves as a bridge between scientific knowledge and regulatory requirements. Did minor flaws in a new drug reveal major. What Is Regulatory Medical Writing.
From www.slideserve.com
PPT WRITING FOR MEDICAL DEVICE COMPANIES THE ROLE OF REGULATORY What Is Regulatory Medical Writing It involves the creation of various. When it comes to writing in the medical field, there are two distinct styles: Did minor flaws in a new drug reveal major flaws in company publication practices? Medical writing is a quarterly publication that aims to educate. Regulatory medical writing serves as a bridge between scientific knowledge and regulatory requirements. What is regulatory. What Is Regulatory Medical Writing.
From cromospharma.com
Regulatory & Publication medical writing Cromos Pharma What Is Regulatory Medical Writing Did minor flaws in a new drug reveal major flaws in company publication practices? Regulatory medical writing serves as a bridge between scientific knowledge and regulatory requirements. It involves the creation of various. Regulatory writing generally covers the gamut of documents that are involved in the drug or medical device development process, ranging from clinical trial level documents (protocols,. Medical. What Is Regulatory Medical Writing.
From pharmaw.com
PharmaWrite What Is Regulatory Medical Writing What is regulatory medical writing? Did minor flaws in a new drug reveal major flaws in company publication practices? When it comes to writing in the medical field, there are two distinct styles: Regulatory medical writing is a specialized domain within the broader field of medical writing, focusing on creating documentation. Regulatory writing generally covers the gamut of documents that. What Is Regulatory Medical Writing.
From www.slideserve.com
PPT AUTOMATION IN REGULATORY MEDICAL WRITING PowerPoint Presentation What Is Regulatory Medical Writing Regulatory writing is primarily concerned with addressing the needs of regulatory agencies and reviewers who are tasked with determining whether a drug or medical device meets the. Did minor flaws in a new drug reveal major flaws in company publication practices? In the dynamic landscape of the healthcare industry, where innovation is constant and regulations are stringent, regulatory medical writing. What Is Regulatory Medical Writing.
From www.pinterest.com
Medical writing for regulatory submission in clinical research and its What Is Regulatory Medical Writing Regulatory medical writing serves as a bridge between scientific knowledge and regulatory requirements. Regulatory medical writing means creating the documentation that regulatory agencies require in the approval process for drugs, devices and. Regulatory writing generally covers the gamut of documents that are involved in the drug or medical device development process, ranging from clinical trial level documents (protocols,. Did minor. What Is Regulatory Medical Writing.
From www.imcgrupo.com
How Regulatory Writing in the Medical Industry Can Help You Avoid What Is Regulatory Medical Writing It involves the creation of various. Regulatory writing generally covers the gamut of documents that are involved in the drug or medical device development process, ranging from clinical trial level documents (protocols,. Regulatory medical writing is a specialized domain within the broader field of medical writing, focusing on creating documentation. Did minor flaws in a new drug reveal major flaws. What Is Regulatory Medical Writing.
From www.smiqgen.com
AUTOMATION (RPA AND AI) IN REGULATORY MEDICAL WRITING AN OVERVIEW What Is Regulatory Medical Writing Regulatory writing is primarily concerned with addressing the needs of regulatory agencies and reviewers who are tasked with determining whether a drug or medical device meets the. Did minor flaws in a new drug reveal major flaws in company publication practices? Regulatory medical writing serves as a bridge between scientific knowledge and regulatory requirements. Regulatory medical writing means creating the. What Is Regulatory Medical Writing.
From www.pinterest.com
Regulatory Medical Writing Bundle RAPS Writing bundle, Medical What Is Regulatory Medical Writing It involves the creation of various. Medical writing is a quarterly publication that aims to educate. When it comes to writing in the medical field, there are two distinct styles: Regulatory medical writing means creating the documentation that regulatory agencies require in the approval process for drugs, devices and. Regulatory writing is primarily concerned with addressing the needs of regulatory. What Is Regulatory Medical Writing.
From www.slideserve.com
PPT WRITING FOR MEDICAL DEVICE COMPANIES THE ROLE OF REGULATORY What Is Regulatory Medical Writing Regulatory medical writing means creating the documentation that regulatory agencies require in the approval process for drugs, devices and. It involves the creation of various. Regulatory writing is primarily concerned with addressing the needs of regulatory agencies and reviewers who are tasked with determining whether a drug or medical device meets the. In the dynamic landscape of the healthcare industry,. What Is Regulatory Medical Writing.
From www.pinterest.com
Regulatory Medical Writing Services in Clinical Research Pepgra What Is Regulatory Medical Writing Did minor flaws in a new drug reveal major flaws in company publication practices? What is regulatory medical writing? Regulatory medical writing serves as a bridge between scientific knowledge and regulatory requirements. Regulatory medical writing is a specialized domain within the broader field of medical writing, focusing on creating documentation. Regulatory writing generally covers the gamut of documents that are. What Is Regulatory Medical Writing.
From www.slideshare.net
What is Regulatory Medical Writing? PDF What Is Regulatory Medical Writing Did minor flaws in a new drug reveal major flaws in company publication practices? When it comes to writing in the medical field, there are two distinct styles: In the dynamic landscape of the healthcare industry, where innovation is constant and regulations are stringent, regulatory medical writing stands as a. Regulatory writing is primarily concerned with addressing the needs of. What Is Regulatory Medical Writing.
From www.slideserve.com
PPT AUTOMATION IN REGULATORY MEDICAL WRITING PowerPoint Presentation What Is Regulatory Medical Writing Regulatory medical writing serves as a bridge between scientific knowledge and regulatory requirements. It involves the creation of various. When it comes to writing in the medical field, there are two distinct styles: What is regulatory medical writing? Regulatory writing is primarily concerned with addressing the needs of regulatory agencies and reviewers who are tasked with determining whether a drug. What Is Regulatory Medical Writing.