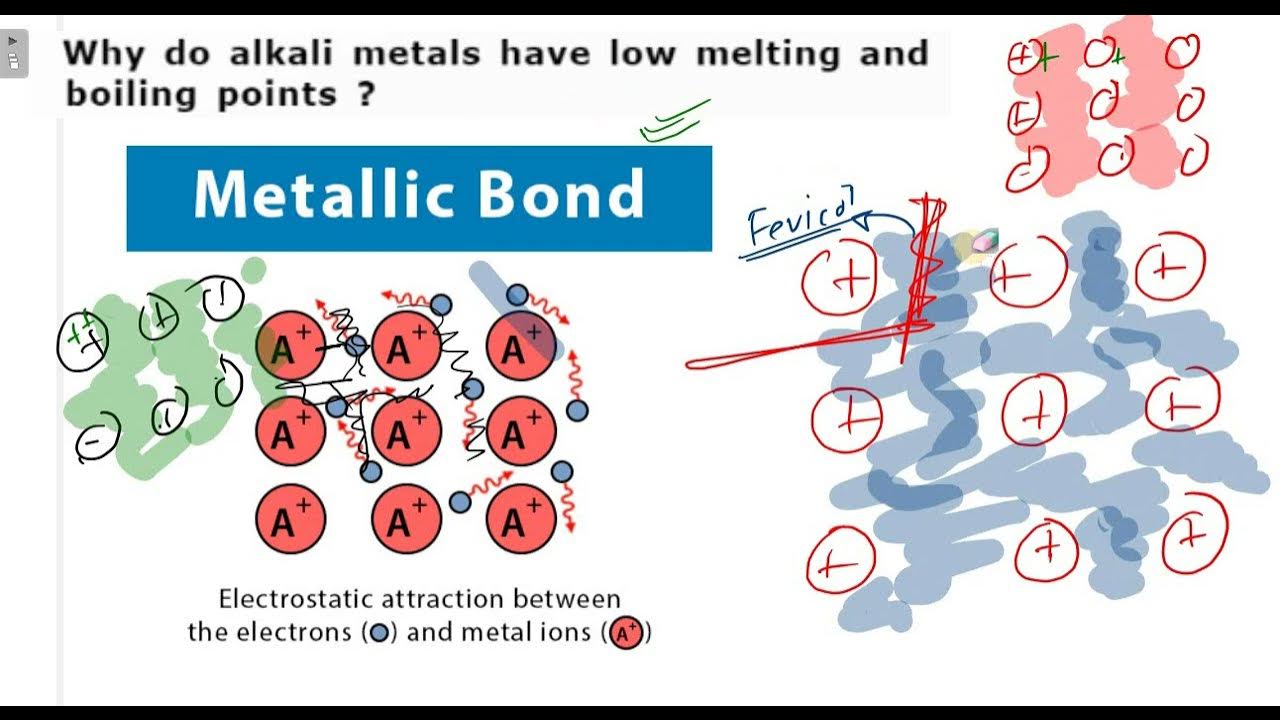
Why Do Alkali Metals Have Low Boiling Points . Alloys of alkali metals exist that melt as low. You have to understand that melting point is related to the bonds in a metal between the metal cation and the 'sea of electrons'. Due to this reason, alkali metals have low melting and boiling points. The group 1 elements are all soft, reactive metals with low melting points. They have low melting points (increasing up the group from 28 c for cs to 180 c for li, whereas typical metals have much higher. Alkali metals have only one valence electron per metal atom and therefore, the energy binding. When any of the group 1 metals is melted, the metallic bond is weakened enough for the atoms to move more freely,. The alkali metals have low melting points, ranging from a high of 179 c (354 f) for lithium to a low of 28.5 c (83.3 f) for cesium. Alkali metals (li, na, k, rb, cs) are soft and have low melting and boiling points. Both the melting and boiling points decrease down the group. Boiling and melting points of alkali metals decrease down the group due to. They react with water to produce an alkaline metal.
from www.youtube.com
Both the melting and boiling points decrease down the group. The alkali metals have low melting points, ranging from a high of 179 c (354 f) for lithium to a low of 28.5 c (83.3 f) for cesium. Alkali metals have only one valence electron per metal atom and therefore, the energy binding. Due to this reason, alkali metals have low melting and boiling points. The group 1 elements are all soft, reactive metals with low melting points. Alloys of alkali metals exist that melt as low. Alkali metals (li, na, k, rb, cs) are soft and have low melting and boiling points. You have to understand that melting point is related to the bonds in a metal between the metal cation and the 'sea of electrons'. When any of the group 1 metals is melted, the metallic bond is weakened enough for the atoms to move more freely,. Boiling and melting points of alkali metals decrease down the group due to.
Why do alkali metals have low melting and boiling points ? YouTube
Why Do Alkali Metals Have Low Boiling Points Alkali metals have only one valence electron per metal atom and therefore, the energy binding. They have low melting points (increasing up the group from 28 c for cs to 180 c for li, whereas typical metals have much higher. Boiling and melting points of alkali metals decrease down the group due to. The group 1 elements are all soft, reactive metals with low melting points. Alloys of alkali metals exist that melt as low. Alkali metals have only one valence electron per metal atom and therefore, the energy binding. Alkali metals (li, na, k, rb, cs) are soft and have low melting and boiling points. Both the melting and boiling points decrease down the group. You have to understand that melting point is related to the bonds in a metal between the metal cation and the 'sea of electrons'. The alkali metals have low melting points, ranging from a high of 179 c (354 f) for lithium to a low of 28.5 c (83.3 f) for cesium. They react with water to produce an alkaline metal. When any of the group 1 metals is melted, the metallic bond is weakened enough for the atoms to move more freely,. Due to this reason, alkali metals have low melting and boiling points.
From www.slideserve.com
PPT Chapter 7 Periodic Properties of the Elements PowerPoint Why Do Alkali Metals Have Low Boiling Points You have to understand that melting point is related to the bonds in a metal between the metal cation and the 'sea of electrons'. Boiling and melting points of alkali metals decrease down the group due to. Alkali metals have only one valence electron per metal atom and therefore, the energy binding. When any of the group 1 metals is. Why Do Alkali Metals Have Low Boiling Points.
From byjus.com
Alkali Metals Properties, Electronic Configuration, Periodic Trends Why Do Alkali Metals Have Low Boiling Points They have low melting points (increasing up the group from 28 c for cs to 180 c for li, whereas typical metals have much higher. Alkali metals (li, na, k, rb, cs) are soft and have low melting and boiling points. Boiling and melting points of alkali metals decrease down the group due to. The alkali metals have low melting. Why Do Alkali Metals Have Low Boiling Points.
From slideplayer.com
Alkali Metals Electrostructure and reactivity Physical properties ppt Why Do Alkali Metals Have Low Boiling Points The alkali metals have low melting points, ranging from a high of 179 c (354 f) for lithium to a low of 28.5 c (83.3 f) for cesium. The group 1 elements are all soft, reactive metals with low melting points. They have low melting points (increasing up the group from 28 c for cs to 180 c for li,. Why Do Alkali Metals Have Low Boiling Points.
From studylib.net
Alkali metals Why Do Alkali Metals Have Low Boiling Points Due to this reason, alkali metals have low melting and boiling points. They react with water to produce an alkaline metal. Boiling and melting points of alkali metals decrease down the group due to. The alkali metals have low melting points, ranging from a high of 179 c (354 f) for lithium to a low of 28.5 c (83.3 f). Why Do Alkali Metals Have Low Boiling Points.
From en.ppt-online.org
The alkali metals online presentation Why Do Alkali Metals Have Low Boiling Points Boiling and melting points of alkali metals decrease down the group due to. They have low melting points (increasing up the group from 28 c for cs to 180 c for li, whereas typical metals have much higher. The group 1 elements are all soft, reactive metals with low melting points. They react with water to produce an alkaline metal.. Why Do Alkali Metals Have Low Boiling Points.
From www.slideserve.com
PPT Melting Points of Alkali Metals PowerPoint Presentation, free Why Do Alkali Metals Have Low Boiling Points They react with water to produce an alkaline metal. Alkali metals have only one valence electron per metal atom and therefore, the energy binding. Alloys of alkali metals exist that melt as low. Due to this reason, alkali metals have low melting and boiling points. The group 1 elements are all soft, reactive metals with low melting points. When any. Why Do Alkali Metals Have Low Boiling Points.
From m20131000606.blogspot.com
Chemistry Group 1 Elements Alkali Metals Why Do Alkali Metals Have Low Boiling Points Alkali metals (li, na, k, rb, cs) are soft and have low melting and boiling points. They have low melting points (increasing up the group from 28 c for cs to 180 c for li, whereas typical metals have much higher. Alkali metals have only one valence electron per metal atom and therefore, the energy binding. Both the melting and. Why Do Alkali Metals Have Low Boiling Points.
From slideplayer.com
Year 9 CHEMISTRY. ppt download Why Do Alkali Metals Have Low Boiling Points Both the melting and boiling points decrease down the group. The alkali metals have low melting points, ranging from a high of 179 c (354 f) for lithium to a low of 28.5 c (83.3 f) for cesium. The group 1 elements are all soft, reactive metals with low melting points. Boiling and melting points of alkali metals decrease down. Why Do Alkali Metals Have Low Boiling Points.
From www.slideserve.com
PPT Group 1 The alkali metals PowerPoint Presentation ID5525387 Why Do Alkali Metals Have Low Boiling Points They react with water to produce an alkaline metal. Alloys of alkali metals exist that melt as low. Due to this reason, alkali metals have low melting and boiling points. The alkali metals have low melting points, ranging from a high of 179 c (354 f) for lithium to a low of 28.5 c (83.3 f) for cesium. The group. Why Do Alkali Metals Have Low Boiling Points.
From byjus.com
Order of boiling point and density for alkali metals Explain Why Do Alkali Metals Have Low Boiling Points You have to understand that melting point is related to the bonds in a metal between the metal cation and the 'sea of electrons'. When any of the group 1 metals is melted, the metallic bond is weakened enough for the atoms to move more freely,. Due to this reason, alkali metals have low melting and boiling points. Alloys of. Why Do Alkali Metals Have Low Boiling Points.
From www.youtube.com
Alkali metals trends, melting and boiling points, uses and Why Do Alkali Metals Have Low Boiling Points Boiling and melting points of alkali metals decrease down the group due to. When any of the group 1 metals is melted, the metallic bond is weakened enough for the atoms to move more freely,. You have to understand that melting point is related to the bonds in a metal between the metal cation and the 'sea of electrons'. The. Why Do Alkali Metals Have Low Boiling Points.
From www.goodscience.com.au
The Periodic Table Good Science Why Do Alkali Metals Have Low Boiling Points Alloys of alkali metals exist that melt as low. When any of the group 1 metals is melted, the metallic bond is weakened enough for the atoms to move more freely,. Both the melting and boiling points decrease down the group. They react with water to produce an alkaline metal. They have low melting points (increasing up the group from. Why Do Alkali Metals Have Low Boiling Points.
From www.slideserve.com
PPT Melting Points of Alkali Metals PowerPoint Presentation, free Why Do Alkali Metals Have Low Boiling Points Alkali metals (li, na, k, rb, cs) are soft and have low melting and boiling points. When any of the group 1 metals is melted, the metallic bond is weakened enough for the atoms to move more freely,. Boiling and melting points of alkali metals decrease down the group due to. Alkali metals have only one valence electron per metal. Why Do Alkali Metals Have Low Boiling Points.
From www.slideserve.com
PPT Where are the alkali metals? PowerPoint Presentation ID5525447 Why Do Alkali Metals Have Low Boiling Points Due to this reason, alkali metals have low melting and boiling points. When any of the group 1 metals is melted, the metallic bond is weakened enough for the atoms to move more freely,. The group 1 elements are all soft, reactive metals with low melting points. The alkali metals have low melting points, ranging from a high of 179. Why Do Alkali Metals Have Low Boiling Points.
From online-learning-college.com
Group 1 alkali metals Properties of alkali metals Reactions Why Do Alkali Metals Have Low Boiling Points Alkali metals (li, na, k, rb, cs) are soft and have low melting and boiling points. The group 1 elements are all soft, reactive metals with low melting points. When any of the group 1 metals is melted, the metallic bond is weakened enough for the atoms to move more freely,. They have low melting points (increasing up the group. Why Do Alkali Metals Have Low Boiling Points.
From cabinet.matttroy.net
Low Melting Point Metals Periodic Table Matttroy Why Do Alkali Metals Have Low Boiling Points Due to this reason, alkali metals have low melting and boiling points. Both the melting and boiling points decrease down the group. The alkali metals have low melting points, ranging from a high of 179 c (354 f) for lithium to a low of 28.5 c (83.3 f) for cesium. The group 1 elements are all soft, reactive metals with. Why Do Alkali Metals Have Low Boiling Points.
From studymind.co.uk
The Transition Metals (GCSE Chemistry) Study Mind Why Do Alkali Metals Have Low Boiling Points Alkali metals have only one valence electron per metal atom and therefore, the energy binding. Alkali metals (li, na, k, rb, cs) are soft and have low melting and boiling points. When any of the group 1 metals is melted, the metallic bond is weakened enough for the atoms to move more freely,. They have low melting points (increasing up. Why Do Alkali Metals Have Low Boiling Points.
From brainly.com
This graph shows the melting and boiling points of the alkali metals Why Do Alkali Metals Have Low Boiling Points Alkali metals (li, na, k, rb, cs) are soft and have low melting and boiling points. Alkali metals have only one valence electron per metal atom and therefore, the energy binding. Boiling and melting points of alkali metals decrease down the group due to. You have to understand that melting point is related to the bonds in a metal between. Why Do Alkali Metals Have Low Boiling Points.
From www.slideserve.com
PPT Group 1 The alkali metals PowerPoint Presentation ID5525387 Why Do Alkali Metals Have Low Boiling Points Alloys of alkali metals exist that melt as low. Both the melting and boiling points decrease down the group. The group 1 elements are all soft, reactive metals with low melting points. The alkali metals have low melting points, ranging from a high of 179 c (354 f) for lithium to a low of 28.5 c (83.3 f) for cesium.. Why Do Alkali Metals Have Low Boiling Points.
From slideplayer.com
Chapter 7 Periodic Properties of the Elements ppt download Why Do Alkali Metals Have Low Boiling Points Boiling and melting points of alkali metals decrease down the group due to. The alkali metals have low melting points, ranging from a high of 179 c (354 f) for lithium to a low of 28.5 c (83.3 f) for cesium. Both the melting and boiling points decrease down the group. Due to this reason, alkali metals have low melting. Why Do Alkali Metals Have Low Boiling Points.
From www.pinterest.com
Alkali metals float on water Alkali metal, Floating in water Why Do Alkali Metals Have Low Boiling Points Alkali metals have only one valence electron per metal atom and therefore, the energy binding. When any of the group 1 metals is melted, the metallic bond is weakened enough for the atoms to move more freely,. They react with water to produce an alkaline metal. They have low melting points (increasing up the group from 28 c for cs. Why Do Alkali Metals Have Low Boiling Points.
From www.youtube.com
Why do alkali metals have low melting and boiling points ? YouTube Why Do Alkali Metals Have Low Boiling Points Due to this reason, alkali metals have low melting and boiling points. They have low melting points (increasing up the group from 28 c for cs to 180 c for li, whereas typical metals have much higher. Alloys of alkali metals exist that melt as low. When any of the group 1 metals is melted, the metallic bond is weakened. Why Do Alkali Metals Have Low Boiling Points.
From www.slideserve.com
PPT Atomic Theory PowerPoint Presentation, free download ID5435746 Why Do Alkali Metals Have Low Boiling Points The alkali metals have low melting points, ranging from a high of 179 c (354 f) for lithium to a low of 28.5 c (83.3 f) for cesium. Alkali metals have only one valence electron per metal atom and therefore, the energy binding. They have low melting points (increasing up the group from 28 c for cs to 180 c. Why Do Alkali Metals Have Low Boiling Points.
From issuu.com
Chemistry Alkali Metals by AshfordSchool Issuu Why Do Alkali Metals Have Low Boiling Points The alkali metals have low melting points, ranging from a high of 179 c (354 f) for lithium to a low of 28.5 c (83.3 f) for cesium. Alkali metals (li, na, k, rb, cs) are soft and have low melting and boiling points. When any of the group 1 metals is melted, the metallic bond is weakened enough for. Why Do Alkali Metals Have Low Boiling Points.
From infogram.com
Top 10 Elements with the lowest boiling point Infogram Why Do Alkali Metals Have Low Boiling Points Alkali metals (li, na, k, rb, cs) are soft and have low melting and boiling points. The group 1 elements are all soft, reactive metals with low melting points. Boiling and melting points of alkali metals decrease down the group due to. They have low melting points (increasing up the group from 28 c for cs to 180 c for. Why Do Alkali Metals Have Low Boiling Points.
From www.nagwa.com
Question Video Identifying Which Alkali Metal Has the Lowest Melting Why Do Alkali Metals Have Low Boiling Points The group 1 elements are all soft, reactive metals with low melting points. They react with water to produce an alkaline metal. Boiling and melting points of alkali metals decrease down the group due to. When any of the group 1 metals is melted, the metallic bond is weakened enough for the atoms to move more freely,. They have low. Why Do Alkali Metals Have Low Boiling Points.
From byjus.com
The alkali metals are low melting. Which of the following alkali metal Why Do Alkali Metals Have Low Boiling Points You have to understand that melting point is related to the bonds in a metal between the metal cation and the 'sea of electrons'. When any of the group 1 metals is melted, the metallic bond is weakened enough for the atoms to move more freely,. Both the melting and boiling points decrease down the group. They have low melting. Why Do Alkali Metals Have Low Boiling Points.
From www.nagwa.com
Question Video Determining Why Covalent Compounds Have Low Melting and Why Do Alkali Metals Have Low Boiling Points They react with water to produce an alkaline metal. When any of the group 1 metals is melted, the metallic bond is weakened enough for the atoms to move more freely,. The alkali metals have low melting points, ranging from a high of 179 c (354 f) for lithium to a low of 28.5 c (83.3 f) for cesium. The. Why Do Alkali Metals Have Low Boiling Points.
From www.adevos.science
The Alkali Metals Periodic Table Adevoscience Why Do Alkali Metals Have Low Boiling Points The alkali metals have low melting points, ranging from a high of 179 c (354 f) for lithium to a low of 28.5 c (83.3 f) for cesium. Alloys of alkali metals exist that melt as low. Alkali metals (li, na, k, rb, cs) are soft and have low melting and boiling points. They have low melting points (increasing up. Why Do Alkali Metals Have Low Boiling Points.
From wagine.com
17 Metals With the Highest Melting Points (and Why) Materials Science Why Do Alkali Metals Have Low Boiling Points Alkali metals (li, na, k, rb, cs) are soft and have low melting and boiling points. You have to understand that melting point is related to the bonds in a metal between the metal cation and the 'sea of electrons'. Boiling and melting points of alkali metals decrease down the group due to. Due to this reason, alkali metals have. Why Do Alkali Metals Have Low Boiling Points.
From www.studocu.com
Alkalimetals gen chem 1 Alkali metals The metals boiling and Why Do Alkali Metals Have Low Boiling Points You have to understand that melting point is related to the bonds in a metal between the metal cation and the 'sea of electrons'. Due to this reason, alkali metals have low melting and boiling points. Alkali metals have only one valence electron per metal atom and therefore, the energy binding. Alloys of alkali metals exist that melt as low.. Why Do Alkali Metals Have Low Boiling Points.
From www.doubtnut.com
[Tamil] Why alkali metals have low melting and boiling points? Why Do Alkali Metals Have Low Boiling Points Alkali metals (li, na, k, rb, cs) are soft and have low melting and boiling points. You have to understand that melting point is related to the bonds in a metal between the metal cation and the 'sea of electrons'. They react with water to produce an alkaline metal. Both the melting and boiling points decrease down the group. They. Why Do Alkali Metals Have Low Boiling Points.
From www.britannica.com
alkali metal Definition, Properties, & Facts Britannica Why Do Alkali Metals Have Low Boiling Points Due to this reason, alkali metals have low melting and boiling points. Both the melting and boiling points decrease down the group. Alloys of alkali metals exist that melt as low. The group 1 elements are all soft, reactive metals with low melting points. They react with water to produce an alkaline metal. They have low melting points (increasing up. Why Do Alkali Metals Have Low Boiling Points.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 2.1.1 Group 1 (Alkali Metals)翰林国际教育 Why Do Alkali Metals Have Low Boiling Points Alloys of alkali metals exist that melt as low. The alkali metals have low melting points, ranging from a high of 179 c (354 f) for lithium to a low of 28.5 c (83.3 f) for cesium. Due to this reason, alkali metals have low melting and boiling points. Both the melting and boiling points decrease down the group. They. Why Do Alkali Metals Have Low Boiling Points.
From www.slideserve.com
PPT Where are the alkali metals? PowerPoint Presentation ID5525447 Why Do Alkali Metals Have Low Boiling Points The alkali metals have low melting points, ranging from a high of 179 c (354 f) for lithium to a low of 28.5 c (83.3 f) for cesium. Alkali metals have only one valence electron per metal atom and therefore, the energy binding. Due to this reason, alkali metals have low melting and boiling points. Alloys of alkali metals exist. Why Do Alkali Metals Have Low Boiling Points.