Chlorine Preparation Formula . The effectiveness of prepared chlorine solutions for use in water hygiene procedures is dependent upon the concentration of the active form of. This information paper provides guidance for preparing high concentrations of a chlorine solution for use as a disinfectant and. Any concentration can be used to make a dilute chlorine solution by applying the. A 1% solution contains 10 g of chlorine per litre = 10 000 mg/litre or 10 000 ppm (parts per million). To prepare a 0.2% chlorine solution the concentration of the bleach to be used, expressed in “active chlorine” on the commercial product,. A single covalent bond connecting the atoms. Chlorine in liquid bleach comes in different concentrations. Avoid skin contact with any of the chemical.
from www.vecteezy.com
Chlorine in liquid bleach comes in different concentrations. A 1% solution contains 10 g of chlorine per litre = 10 000 mg/litre or 10 000 ppm (parts per million). Any concentration can be used to make a dilute chlorine solution by applying the. This information paper provides guidance for preparing high concentrations of a chlorine solution for use as a disinfectant and. To prepare a 0.2% chlorine solution the concentration of the bleach to be used, expressed in “active chlorine” on the commercial product,. A single covalent bond connecting the atoms. The effectiveness of prepared chlorine solutions for use in water hygiene procedures is dependent upon the concentration of the active form of. Avoid skin contact with any of the chemical.
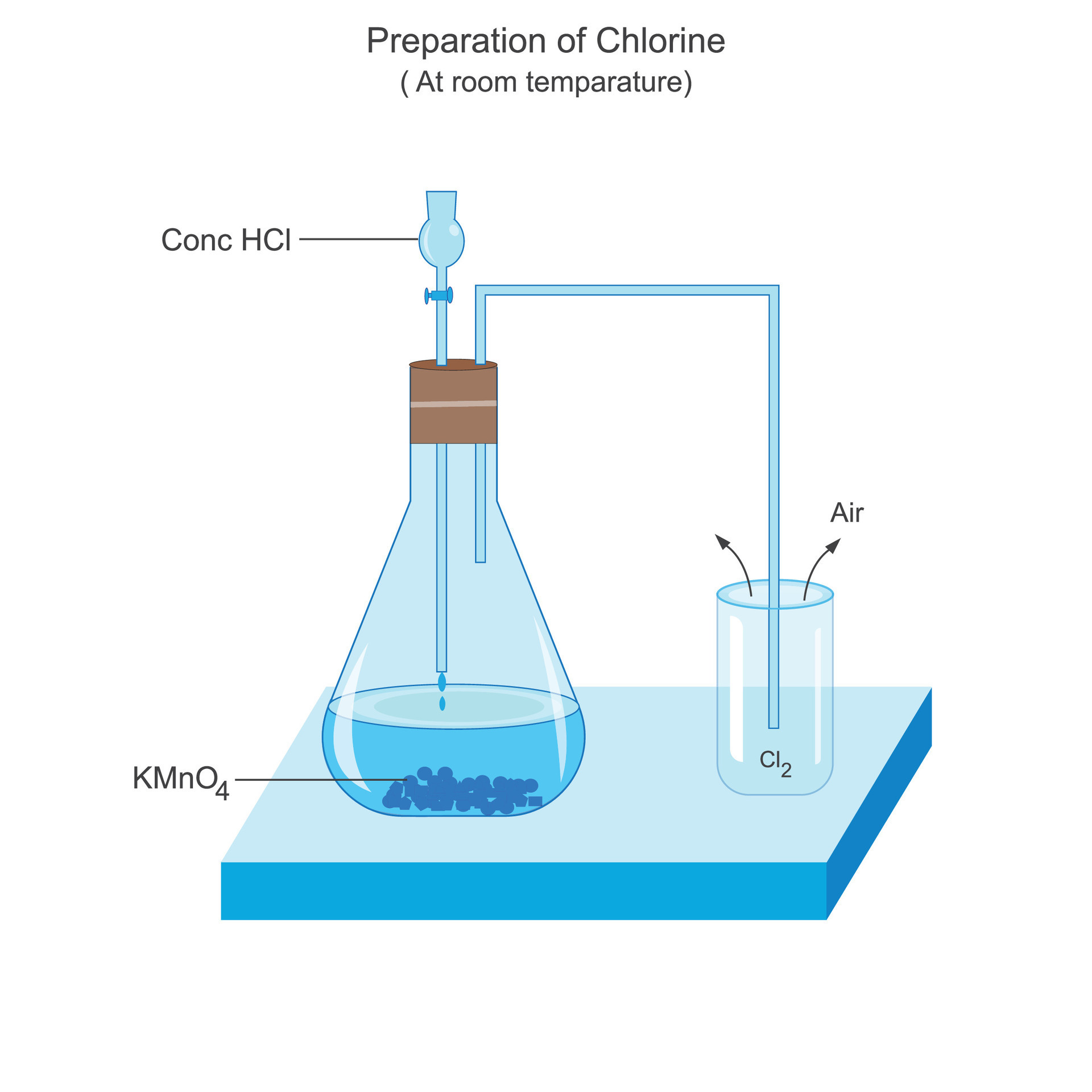
Preparation of chlorine at room temparature in laboratory. vector image
Chlorine Preparation Formula Chlorine in liquid bleach comes in different concentrations. This information paper provides guidance for preparing high concentrations of a chlorine solution for use as a disinfectant and. Avoid skin contact with any of the chemical. To prepare a 0.2% chlorine solution the concentration of the bleach to be used, expressed in “active chlorine” on the commercial product,. Any concentration can be used to make a dilute chlorine solution by applying the. The effectiveness of prepared chlorine solutions for use in water hygiene procedures is dependent upon the concentration of the active form of. A single covalent bond connecting the atoms. A 1% solution contains 10 g of chlorine per litre = 10 000 mg/litre or 10 000 ppm (parts per million). Chlorine in liquid bleach comes in different concentrations.
From www.youtube.com
Preparation Of Chlorine And It's Properties ( Part 2 ) YouTube Chlorine Preparation Formula To prepare a 0.2% chlorine solution the concentration of the bleach to be used, expressed in “active chlorine” on the commercial product,. A 1% solution contains 10 g of chlorine per litre = 10 000 mg/litre or 10 000 ppm (parts per million). The effectiveness of prepared chlorine solutions for use in water hygiene procedures is dependent upon the concentration. Chlorine Preparation Formula.
From www.youtube.com
How to Write the Formula for Chlorine monoxide YouTube Chlorine Preparation Formula This information paper provides guidance for preparing high concentrations of a chlorine solution for use as a disinfectant and. To prepare a 0.2% chlorine solution the concentration of the bleach to be used, expressed in “active chlorine” on the commercial product,. Any concentration can be used to make a dilute chlorine solution by applying the. A 1% solution contains 10. Chlorine Preparation Formula.
From www.alamy.com
Molecular Model of Chlorine (Cl2) Molecule. Vector Illustration Stock Chlorine Preparation Formula Any concentration can be used to make a dilute chlorine solution by applying the. The effectiveness of prepared chlorine solutions for use in water hygiene procedures is dependent upon the concentration of the active form of. This information paper provides guidance for preparing high concentrations of a chlorine solution for use as a disinfectant and. To prepare a 0.2% chlorine. Chlorine Preparation Formula.
From www.coursehero.com
[Solved] Draw the structure of the alkyl chloride needed to prepare Chlorine Preparation Formula The effectiveness of prepared chlorine solutions for use in water hygiene procedures is dependent upon the concentration of the active form of. To prepare a 0.2% chlorine solution the concentration of the bleach to be used, expressed in “active chlorine” on the commercial product,. A 1% solution contains 10 g of chlorine per litre = 10 000 mg/litre or 10. Chlorine Preparation Formula.
From www.examples.com
Chlorine (Cl) Definition, Preparation, Properties, Uses, Compounds Chlorine Preparation Formula A single covalent bond connecting the atoms. To prepare a 0.2% chlorine solution the concentration of the bleach to be used, expressed in “active chlorine” on the commercial product,. Any concentration can be used to make a dilute chlorine solution by applying the. Chlorine in liquid bleach comes in different concentrations. Avoid skin contact with any of the chemical. A. Chlorine Preparation Formula.
From www.youtube.com
Ammonium Chloride method of preparation, Physical, chemical properties Chlorine Preparation Formula Chlorine in liquid bleach comes in different concentrations. The effectiveness of prepared chlorine solutions for use in water hygiene procedures is dependent upon the concentration of the active form of. This information paper provides guidance for preparing high concentrations of a chlorine solution for use as a disinfectant and. A single covalent bond connecting the atoms. A 1% solution contains. Chlorine Preparation Formula.
From testbook.com
Iron (III) Chloride Formula Know Structure, Preparation,& Uses Chlorine Preparation Formula The effectiveness of prepared chlorine solutions for use in water hygiene procedures is dependent upon the concentration of the active form of. To prepare a 0.2% chlorine solution the concentration of the bleach to be used, expressed in “active chlorine” on the commercial product,. Any concentration can be used to make a dilute chlorine solution by applying the. Avoid skin. Chlorine Preparation Formula.
From www.shutterstock.com
Structural Chemical Formula Chlorine Medical Infographic Stock Vector Chlorine Preparation Formula A 1% solution contains 10 g of chlorine per litre = 10 000 mg/litre or 10 000 ppm (parts per million). Any concentration can be used to make a dilute chlorine solution by applying the. The effectiveness of prepared chlorine solutions for use in water hygiene procedures is dependent upon the concentration of the active form of. This information paper. Chlorine Preparation Formula.
From mungfali.com
Acid Chloride Synthesis Chlorine Preparation Formula This information paper provides guidance for preparing high concentrations of a chlorine solution for use as a disinfectant and. The effectiveness of prepared chlorine solutions for use in water hygiene procedures is dependent upon the concentration of the active form of. Chlorine in liquid bleach comes in different concentrations. A single covalent bond connecting the atoms. Any concentration can be. Chlorine Preparation Formula.
From www.researchgate.net
Formula and calculation for the Preparation of common chlorine Chlorine Preparation Formula A single covalent bond connecting the atoms. A 1% solution contains 10 g of chlorine per litre = 10 000 mg/litre or 10 000 ppm (parts per million). The effectiveness of prepared chlorine solutions for use in water hygiene procedures is dependent upon the concentration of the active form of. This information paper provides guidance for preparing high concentrations of. Chlorine Preparation Formula.
From screening.iarc.fr
Atlas of visual inspection of the cervix with acetic acid for screening Chlorine Preparation Formula This information paper provides guidance for preparing high concentrations of a chlorine solution for use as a disinfectant and. Chlorine in liquid bleach comes in different concentrations. The effectiveness of prepared chlorine solutions for use in water hygiene procedures is dependent upon the concentration of the active form of. Any concentration can be used to make a dilute chlorine solution. Chlorine Preparation Formula.
From stock.adobe.com
Lewis structural formula of chlorine, molecular formula Stock Vector Chlorine Preparation Formula Chlorine in liquid bleach comes in different concentrations. The effectiveness of prepared chlorine solutions for use in water hygiene procedures is dependent upon the concentration of the active form of. A 1% solution contains 10 g of chlorine per litre = 10 000 mg/litre or 10 000 ppm (parts per million). This information paper provides guidance for preparing high concentrations. Chlorine Preparation Formula.
From www.essentialchemicalindustry.org
Chlorine Chlorine Preparation Formula The effectiveness of prepared chlorine solutions for use in water hygiene procedures is dependent upon the concentration of the active form of. Chlorine in liquid bleach comes in different concentrations. A single covalent bond connecting the atoms. Any concentration can be used to make a dilute chlorine solution by applying the. A 1% solution contains 10 g of chlorine per. Chlorine Preparation Formula.
From www.slideserve.com
PPT CLEANLINESS OF THE WARD AND ITS ANNEXES PowerPoint Presentation Chlorine Preparation Formula A single covalent bond connecting the atoms. Chlorine in liquid bleach comes in different concentrations. A 1% solution contains 10 g of chlorine per litre = 10 000 mg/litre or 10 000 ppm (parts per million). To prepare a 0.2% chlorine solution the concentration of the bleach to be used, expressed in “active chlorine” on the commercial product,. Avoid skin. Chlorine Preparation Formula.
From preventepidemics.org
Making Disinfectant Chlorine Solutions Prevent Epidemics Chlorine Preparation Formula To prepare a 0.2% chlorine solution the concentration of the bleach to be used, expressed in “active chlorine” on the commercial product,. Chlorine in liquid bleach comes in different concentrations. This information paper provides guidance for preparing high concentrations of a chlorine solution for use as a disinfectant and. A 1% solution contains 10 g of chlorine per litre =. Chlorine Preparation Formula.
From www.slideserve.com
PPT Preparation of tbutyl chloride PowerPoint Presentation, free Chlorine Preparation Formula The effectiveness of prepared chlorine solutions for use in water hygiene procedures is dependent upon the concentration of the active form of. This information paper provides guidance for preparing high concentrations of a chlorine solution for use as a disinfectant and. Avoid skin contact with any of the chemical. A single covalent bond connecting the atoms. Any concentration can be. Chlorine Preparation Formula.
From icsechemistry16.blogspot.com
Preparation of Iron (III) chloride (Ferric chloride) Chlorine Preparation Formula Chlorine in liquid bleach comes in different concentrations. The effectiveness of prepared chlorine solutions for use in water hygiene procedures is dependent upon the concentration of the active form of. A single covalent bond connecting the atoms. Any concentration can be used to make a dilute chlorine solution by applying the. This information paper provides guidance for preparing high concentrations. Chlorine Preparation Formula.
From www.examples.com
Chlorine (Cl) Definition, Preparation, Properties, Uses, Compounds Chlorine Preparation Formula A single covalent bond connecting the atoms. A 1% solution contains 10 g of chlorine per litre = 10 000 mg/litre or 10 000 ppm (parts per million). Any concentration can be used to make a dilute chlorine solution by applying the. Avoid skin contact with any of the chemical. To prepare a 0.2% chlorine solution the concentration of the. Chlorine Preparation Formula.
From www.slideserve.com
PPT CLEANLINESS OF THE WARD AND ITS ANNEXES PowerPoint Presentation Chlorine Preparation Formula Any concentration can be used to make a dilute chlorine solution by applying the. The effectiveness of prepared chlorine solutions for use in water hygiene procedures is dependent upon the concentration of the active form of. To prepare a 0.2% chlorine solution the concentration of the bleach to be used, expressed in “active chlorine” on the commercial product,. Chlorine in. Chlorine Preparation Formula.
From classnotes.org.in
Chlorine Chemistry, Class 12, The pBlock Elements Chlorine Preparation Formula Avoid skin contact with any of the chemical. A 1% solution contains 10 g of chlorine per litre = 10 000 mg/litre or 10 000 ppm (parts per million). Chlorine in liquid bleach comes in different concentrations. A single covalent bond connecting the atoms. Any concentration can be used to make a dilute chlorine solution by applying the. This information. Chlorine Preparation Formula.
From www.youtube.com
Laboratory Preparation Of Chlorine Using Potassium Permanganate Chlorine Preparation Formula Chlorine in liquid bleach comes in different concentrations. To prepare a 0.2% chlorine solution the concentration of the bleach to be used, expressed in “active chlorine” on the commercial product,. A single covalent bond connecting the atoms. This information paper provides guidance for preparing high concentrations of a chlorine solution for use as a disinfectant and. Any concentration can be. Chlorine Preparation Formula.
From scienceinfo.com
Chloroform Preparation, Properties, Reactions, Uses, Chlorine Preparation Formula Chlorine in liquid bleach comes in different concentrations. A 1% solution contains 10 g of chlorine per litre = 10 000 mg/litre or 10 000 ppm (parts per million). The effectiveness of prepared chlorine solutions for use in water hygiene procedures is dependent upon the concentration of the active form of. This information paper provides guidance for preparing high concentrations. Chlorine Preparation Formula.
From www.slideserve.com
PPT CLEANLINESS OF THE WARD AND ITS ANNEXES PowerPoint Presentation Chlorine Preparation Formula Avoid skin contact with any of the chemical. Any concentration can be used to make a dilute chlorine solution by applying the. A single covalent bond connecting the atoms. This information paper provides guidance for preparing high concentrations of a chlorine solution for use as a disinfectant and. Chlorine in liquid bleach comes in different concentrations. The effectiveness of prepared. Chlorine Preparation Formula.
From www.fanpop.com
Preparation of chlorine (gas) in laboratory Chemistry Fanpop Chlorine Preparation Formula This information paper provides guidance for preparing high concentrations of a chlorine solution for use as a disinfectant and. Any concentration can be used to make a dilute chlorine solution by applying the. A 1% solution contains 10 g of chlorine per litre = 10 000 mg/litre or 10 000 ppm (parts per million). To prepare a 0.2% chlorine solution. Chlorine Preparation Formula.
From www.scribd.com
Chlorine Preparation PDF Chlorine Preparation Formula To prepare a 0.2% chlorine solution the concentration of the bleach to be used, expressed in “active chlorine” on the commercial product,. Any concentration can be used to make a dilute chlorine solution by applying the. Avoid skin contact with any of the chemical. The effectiveness of prepared chlorine solutions for use in water hygiene procedures is dependent upon the. Chlorine Preparation Formula.
From www.youtube.com
How to Balance C + Cl2 = CCl4 (Carbon + Chlorine gas) YouTube Chlorine Preparation Formula To prepare a 0.2% chlorine solution the concentration of the bleach to be used, expressed in “active chlorine” on the commercial product,. A single covalent bond connecting the atoms. Any concentration can be used to make a dilute chlorine solution by applying the. A 1% solution contains 10 g of chlorine per litre = 10 000 mg/litre or 10 000. Chlorine Preparation Formula.
From www.w3schools.blog
Preparation, properties and uses of chlorine and hydrochloric acid Chlorine Preparation Formula The effectiveness of prepared chlorine solutions for use in water hygiene procedures is dependent upon the concentration of the active form of. Any concentration can be used to make a dilute chlorine solution by applying the. Avoid skin contact with any of the chemical. To prepare a 0.2% chlorine solution the concentration of the bleach to be used, expressed in. Chlorine Preparation Formula.
From www.slideserve.com
PPT Chlorine Chemistry PowerPoint Presentation, free download ID Chlorine Preparation Formula A 1% solution contains 10 g of chlorine per litre = 10 000 mg/litre or 10 000 ppm (parts per million). This information paper provides guidance for preparing high concentrations of a chlorine solution for use as a disinfectant and. The effectiveness of prepared chlorine solutions for use in water hygiene procedures is dependent upon the concentration of the active. Chlorine Preparation Formula.
From www.vecteezy.com
Preparation of chlorine at room temparature in laboratory. vector image Chlorine Preparation Formula This information paper provides guidance for preparing high concentrations of a chlorine solution for use as a disinfectant and. Avoid skin contact with any of the chemical. Any concentration can be used to make a dilute chlorine solution by applying the. To prepare a 0.2% chlorine solution the concentration of the bleach to be used, expressed in “active chlorine” on. Chlorine Preparation Formula.
From www.masterorganicchemistry.com
Synthesis (2) Reactions of Alkanes — Master Organic Chemistry Chlorine Preparation Formula A 1% solution contains 10 g of chlorine per litre = 10 000 mg/litre or 10 000 ppm (parts per million). Any concentration can be used to make a dilute chlorine solution by applying the. This information paper provides guidance for preparing high concentrations of a chlorine solution for use as a disinfectant and. Chlorine in liquid bleach comes in. Chlorine Preparation Formula.
From www.examples.com
Chlorine (Cl) Definition, Preparation, Properties, Uses, Compounds Chlorine Preparation Formula Any concentration can be used to make a dilute chlorine solution by applying the. A 1% solution contains 10 g of chlorine per litre = 10 000 mg/litre or 10 000 ppm (parts per million). To prepare a 0.2% chlorine solution the concentration of the bleach to be used, expressed in “active chlorine” on the commercial product,. Avoid skin contact. Chlorine Preparation Formula.
From www.examples.com
Chlorine (Cl) Definition, Preparation, Properties, Uses, Compounds Chlorine Preparation Formula To prepare a 0.2% chlorine solution the concentration of the bleach to be used, expressed in “active chlorine” on the commercial product,. A single covalent bond connecting the atoms. The effectiveness of prepared chlorine solutions for use in water hygiene procedures is dependent upon the concentration of the active form of. Chlorine in liquid bleach comes in different concentrations. Any. Chlorine Preparation Formula.
From www.youtube.com
Iron(iii) Chloride Preparation YouTube Chlorine Preparation Formula Avoid skin contact with any of the chemical. This information paper provides guidance for preparing high concentrations of a chlorine solution for use as a disinfectant and. To prepare a 0.2% chlorine solution the concentration of the bleach to be used, expressed in “active chlorine” on the commercial product,. A 1% solution contains 10 g of chlorine per litre =. Chlorine Preparation Formula.
From www.examples.com
Chlorine (Cl) Definition, Preparation, Properties, Uses, Compounds Chlorine Preparation Formula The effectiveness of prepared chlorine solutions for use in water hygiene procedures is dependent upon the concentration of the active form of. Any concentration can be used to make a dilute chlorine solution by applying the. To prepare a 0.2% chlorine solution the concentration of the bleach to be used, expressed in “active chlorine” on the commercial product,. This information. Chlorine Preparation Formula.
From www.youtube.com
Preparation of Chlorine gas in lab. diagram making video YouTube Chlorine Preparation Formula Avoid skin contact with any of the chemical. Chlorine in liquid bleach comes in different concentrations. To prepare a 0.2% chlorine solution the concentration of the bleach to be used, expressed in “active chlorine” on the commercial product,. The effectiveness of prepared chlorine solutions for use in water hygiene procedures is dependent upon the concentration of the active form of.. Chlorine Preparation Formula.