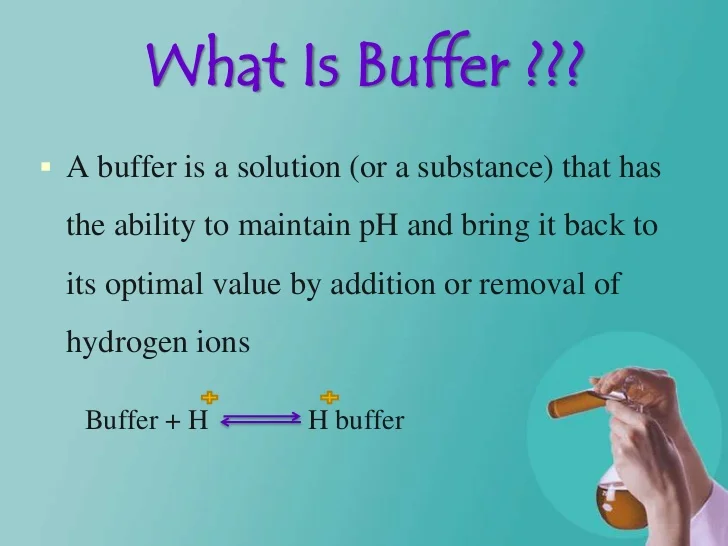
What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer . To understand the mechanism of buffer action, we can take the example of an acidic buffer that is made up of a weak acid like acetic acid and its. An example of a buffer that. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. An acidic buffer solution is simply one which. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. A buffer (or buffered ) solution is one that resists a change. It is able to neutralize small amounts of added acid or base, thus. Buffer action refers to the property of solutions containing an acid and its conjugate base to resist changes in ph when acids or bases are added. A solution of acetic acid (\(\ce{ch3cooh}\) and sodium acetate \(\ce{ch3coona}\)) is an example of a buffer that consists of a weak acid and its salt. The mechanism involves a buffer, a solution that resists dramatic changes in ph.
from www.slideshare.net
Buffer action refers to the property of solutions containing an acid and its conjugate base to resist changes in ph when acids or bases are added. An example of a buffer that. The mechanism involves a buffer, a solution that resists dramatic changes in ph. To understand the mechanism of buffer action, we can take the example of an acidic buffer that is made up of a weak acid like acetic acid and its. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. An acidic buffer solution is simply one which. A buffer (or buffered ) solution is one that resists a change. It is able to neutralize small amounts of added acid or base, thus. A solution of acetic acid (\(\ce{ch3cooh}\) and sodium acetate \(\ce{ch3coona}\)) is an example of a buffer that consists of a weak acid and its salt.
Buffer system
What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer An example of a buffer that. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. An acidic buffer solution is simply one which. An example of a buffer that. A buffer (or buffered ) solution is one that resists a change. The mechanism involves a buffer, a solution that resists dramatic changes in ph. Buffer action refers to the property of solutions containing an acid and its conjugate base to resist changes in ph when acids or bases are added. It is able to neutralize small amounts of added acid or base, thus. To understand the mechanism of buffer action, we can take the example of an acidic buffer that is made up of a weak acid like acetic acid and its. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A solution of acetic acid (\(\ce{ch3cooh}\) and sodium acetate \(\ce{ch3coona}\)) is an example of a buffer that consists of a weak acid and its salt.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer An acidic buffer solution is simply one which. A buffer (or buffered ) solution is one that resists a change. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. A solution of acetic acid (\(\ce{ch3cooh}\) and sodium acetate \(\ce{ch3coona}\)) is an example of a buffer that. What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer.
From courses.lumenlearning.com
Buffers Chemistry What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffer action refers to the property of solutions containing an acid and its conjugate base to resist changes. What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer.
From www.geeksforgeeks.org
Buffer Solution Definition, Types, Formula, Examples, and FAQs What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer An acidic buffer solution is simply one which. A solution of acetic acid (\(\ce{ch3cooh}\) and sodium acetate \(\ce{ch3coona}\)) is an example of a buffer that consists of a weak acid and its salt. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small amounts of. What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer.
From studyx.ai
1 What is a buffer solution Explain mechanism StudyX What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer A solution of acetic acid (\(\ce{ch3cooh}\) and sodium acetate \(\ce{ch3coona}\)) is an example of a buffer that consists of a weak acid and its salt. An example of a buffer that. Buffer action refers to the property of solutions containing an acid and its conjugate base to resist changes in ph when acids or bases are added. An acidic buffer. What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer.
From www.youtube.com
What is Buffer Solution Types of Buffer Solution Acidic Buffer What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. An acidic buffer solution is simply one which. Buffer action refers to the property of solutions containing an acid and its conjugate base to resist changes in ph when acids or bases are added. To understand the mechanism of buffer action,. What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer.
From www.youtube.com
Acidic and Basic buffer Identification and Preparation Tips and Tricks What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. To understand the mechanism of buffer action, we can take the example of an acidic buffer that is made up of a weak acid like acetic acid and its. A buffer (or buffered ) solution is one that resists a change.. What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer.
From design.udlvirtual.edu.pe
What Is A Buffer Solution Design Talk What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer An example of a buffer that. The mechanism involves a buffer, a solution that resists dramatic changes in ph. An acidic buffer solution is simply one which. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. To understand the mechanism of buffer action, we can take. What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer.
From www.pinterest.com
Understanding Buffer Solution Chemistry A Complete Guide What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer An example of a buffer that. A solution of acetic acid (\(\ce{ch3cooh}\) and sodium acetate \(\ce{ch3coona}\)) is an example of a buffer that consists of a weak acid and its salt. An acidic buffer solution is simply one which. The mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer (or buffered ) solution is one. What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer.
From www.slideserve.com
PPT Mechanism of buffer action and buffer preparation PowerPoint What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A solution of acetic acid (\(\ce{ch3cooh}\) and sodium acetate \(\ce{ch3coona}\)) is an example of a buffer that consists of a weak acid and its salt. To understand the mechanism of buffer action, we can take the example of an acidic buffer. What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer It is able to neutralize small amounts of added acid or base, thus. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. To understand the mechanism of. What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer.
From id.pinterest.com
buffer solution , Acidic & basic buffer,weak acid +salt of weak acid What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer To understand the mechanism of buffer action, we can take the example of an acidic buffer that is made up of a weak acid like acetic acid and its. An acidic buffer solution is simply one which. A buffer (or buffered ) solution is one that resists a change. A solution of acetic acid (\(\ce{ch3cooh}\) and sodium acetate \(\ce{ch3coona}\)) is. What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer.
From slidetodoc.com
Mechanism of buffer action and buffer preparation Maintaining What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer A buffer (or buffered ) solution is one that resists a change. The mechanism involves a buffer, a solution that resists dramatic changes in ph. It is able to neutralize small amounts of added acid or base, thus. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution. What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer.
From chemistrypubs.com
Buffer Solution Definition, Examples, Mechanisms, Preparations What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. It is able to neutralize small amounts of added acid or base, thus. An acidic buffer solution is simply one which. An example of a buffer that. Buffer action refers to the property of solutions containing an. What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer.
From www.aakash.ac.in
Acidic Buffer Solution Definition, Calculation & Applications What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The mechanism involves a buffer, a solution that resists dramatic changes in ph. Buffer action refers to the property of solutions containing an acid and its conjugate base to resist changes in ph when acids or bases are added. To understand. What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer.
From www.slideserve.com
PPT Blood Gases, pH and Buffer system PowerPoint Presentation ID257615 What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. To understand the mechanism of buffer action, we can take the example of an acidic buffer that is made up of a weak acid like acetic acid and its. A buffer (or buffered ) solution is one that resists a change.. What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer To understand the mechanism of buffer action, we can take the example of an acidic buffer that is made up of a weak acid like acetic acid and its. The mechanism involves a buffer, a solution that resists dramatic changes in ph. A solution of acetic acid (\(\ce{ch3cooh}\) and sodium acetate \(\ce{ch3coona}\)) is an example of a buffer that consists. What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer Buffer action refers to the property of solutions containing an acid and its conjugate base to resist changes in ph when acids or bases are added. A buffer (or buffered ) solution is one that resists a change. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to. What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer.
From mydigitalkemistry.com
What is a buffer solution simple definition? Free Chemistry Learning What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. To understand the mechanism of buffer action, we can take the example of an acidic buffer that is made up of a weak acid like acetic acid and its. A buffer (or buffered ) solution is one that resists a change.. What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer.
From www.slideshare.net
Buffers in chemical analysis, types of buffers What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer A solution of acetic acid (\(\ce{ch3cooh}\) and sodium acetate \(\ce{ch3coona}\)) is an example of a buffer that consists of a weak acid and its salt. An acidic buffer solution is simply one which. Buffer action refers to the property of solutions containing an acid and its conjugate base to resist changes in ph when acids or bases are added. A. What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer.
From chemistrytalk.org
What is a Buffer Solution? Chemistry ChemTalk What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer A solution of acetic acid (\(\ce{ch3cooh}\) and sodium acetate \(\ce{ch3coona}\)) is an example of a buffer that consists of a weak acid and its salt. The mechanism involves a buffer, a solution that resists dramatic changes in ph. It is able to neutralize small amounts of added acid or base, thus. A buffer (or buffered ) solution is one that. What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer.
From www.slideshare.net
Buffer system What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer An example of a buffer that. Buffer action refers to the property of solutions containing an acid and its conjugate base to resist changes in ph when acids or bases are added. A solution of acetic acid (\(\ce{ch3cooh}\) and sodium acetate \(\ce{ch3coona}\)) is an example of a buffer that consists of a weak acid and its salt. A buffer (or. What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer.
From klaknjndj.blob.core.windows.net
How Do Buffers Work Chemistry at Felix Balch blog What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer To understand the mechanism of buffer action, we can take the example of an acidic buffer that is made up of a weak acid like acetic acid and its. An acidic buffer solution is simply one which. The mechanism involves a buffer, a solution that resists dramatic changes in ph. Buffer action refers to the property of solutions containing an. What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer.
From loexxrihd.blob.core.windows.net
What Is The Function Of A Buffer What Is A Buffer Made From at What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer It is able to neutralize small amounts of added acid or base, thus. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer (or buffered ) solution is one that resists a change. To understand the mechanism of buffer action, we can take the example of an acidic buffer. What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID2372473 What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer It is able to neutralize small amounts of added acid or base, thus. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. To understand the mechanism of buffer action, we can take the example of an acidic buffer that is made up of a weak acid like acetic acid and. What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer.
From www.slideshare.net
2012 topic 18 2 buffer solutions What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer It is able to neutralize small amounts of added acid or base, thus. A buffer (or buffered ) solution is one that resists a change. The mechanism involves a buffer, a solution that resists dramatic changes in ph. To understand the mechanism of buffer action, we can take the example of an acidic buffer that is made up of a. What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer.
From www.youtube.com
Buffer Action Acidic and basic buffer Action how buffer resist pH What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to it. Buffer action refers to the property of solutions containing an acid and its conjugate base to resist changes in ph when acids or bases are added. A solution of acetic acid (\(\ce{ch3cooh}\) and sodium acetate \(\ce{ch3coona}\)) is. What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer.
From www.youtube.com
18.2.1 Describe the composition of a buffer solution and explain its What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer An example of a buffer that. The mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution is one which resists changes in ph when small quantities of an acid or an alkali are added to. What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer.
From guides.hostos.cuny.edu
Chapter 11 Acids and Bases CHE 105/110 Introduction to Chemistry What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small amounts of added acid or base, thus. A solution of acetic acid (\(\ce{ch3cooh}\) and sodium acetate \(\ce{ch3coona}\)) is an example of a buffer that consists of a weak acid and its salt. An acidic buffer. What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer.
From www.pinterest.co.kr
Mechanism of Buffer action of an acid buffer Buffer solution What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer An example of a buffer that. Buffer action refers to the property of solutions containing an acid and its conjugate base to resist changes in ph when acids or bases are added. It is able to neutralize small amounts of added acid or base, thus. An acidic buffer solution is simply one which. A solution of acetic acid (\(\ce{ch3cooh}\) and. What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer An acidic buffer solution is simply one which. Buffer action refers to the property of solutions containing an acid and its conjugate base to resist changes in ph when acids or bases are added. An example of a buffer that. The mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer solution is one which resists. What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer.
From www.geeksforgeeks.org
Buffer Solution Definition, Types, Formula, Examples, and FAQs What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer The mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer (or buffered ) solution is one that resists a change. An example of a buffer that. Buffer action refers to the property of solutions containing an. What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer.
From www.slideserve.com
PPT Chem. Concepts Buffers PowerPoint Presentation, free download What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer An acidic buffer solution is simply one which. The mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffer action refers to the property of solutions containing an acid and its conjugate base to resist changes in ph. What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer A solution of acetic acid (\(\ce{ch3cooh}\) and sodium acetate \(\ce{ch3coona}\)) is an example of a buffer that consists of a weak acid and its salt. Buffer action refers to the property of solutions containing an acid and its conjugate base to resist changes in ph when acids or bases are added. A buffer (or buffered ) solution is one that. What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer.
From www.ck12.org
Buffer Solutions Overview ( Video ) Chemistry CK12 Foundation What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer A solution of acetic acid (\(\ce{ch3cooh}\) and sodium acetate \(\ce{ch3coona}\)) is an example of a buffer that consists of a weak acid and its salt. A buffer (or buffered ) solution is one that resists a change. The mechanism involves a buffer, a solution that resists dramatic changes in ph. An example of a buffer that. A buffer is a. What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer To understand the mechanism of buffer action, we can take the example of an acidic buffer that is made up of a weak acid like acetic acid and its. It is able to neutralize small amounts of added acid or base, thus. The mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer is a solution. What Is Buffer Solution Explain The Mechanism Of Buffer Action Of Acidic Buffer.