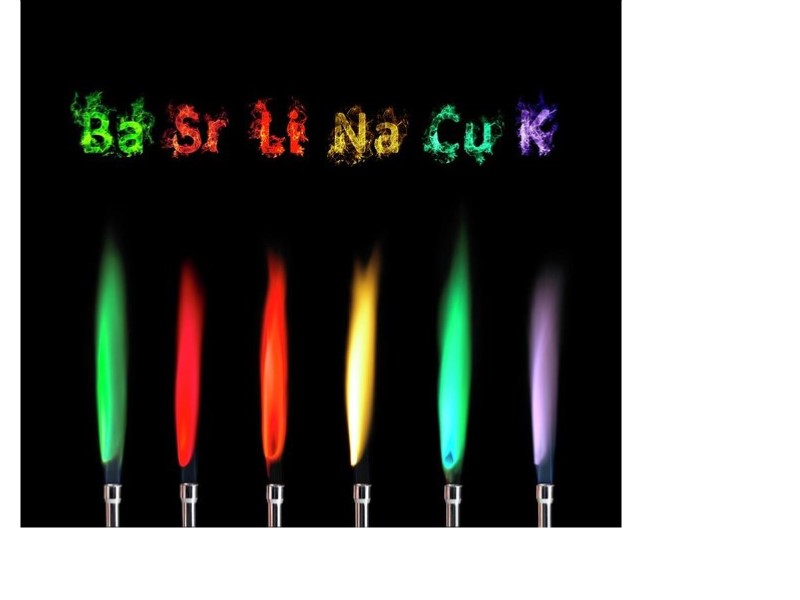
Flame Lab Conclusion . liquids or solids can be tested simply by introducing the sample into the flame. Your instructor will dip a looped wire into one of the solutions supplied, and then hold it in the bunsen burner flame. The premise is that heat gives energy to elements and ions, causing them to emit light at a characteristic color or emission spectrum. in conclusion, this lab experiment aimed to determine the characteristic flame colors of various metallic ions in a flame test and understand the. in conclusion, the flame test lab provided valuable insights into the behavior of metal ions when subjected to. flame test formal lab; Whatever instrument is used to introduce. the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. Flame tests of metal cations. Sc4 (ldc module #1) chemists began studying colored flames in the 18th century and soon used flame.
from chemcats.blogspot.com
Sc4 (ldc module #1) chemists began studying colored flames in the 18th century and soon used flame. Your instructor will dip a looped wire into one of the solutions supplied, and then hold it in the bunsen burner flame. Whatever instrument is used to introduce. in conclusion, the flame test lab provided valuable insights into the behavior of metal ions when subjected to. in conclusion, this lab experiment aimed to determine the characteristic flame colors of various metallic ions in a flame test and understand the. Flame tests of metal cations. the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. liquids or solids can be tested simply by introducing the sample into the flame. flame test formal lab; The premise is that heat gives energy to elements and ions, causing them to emit light at a characteristic color or emission spectrum.
Chemistry Friday Flame Test Lab
Flame Lab Conclusion the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. Sc4 (ldc module #1) chemists began studying colored flames in the 18th century and soon used flame. Whatever instrument is used to introduce. in conclusion, the flame test lab provided valuable insights into the behavior of metal ions when subjected to. liquids or solids can be tested simply by introducing the sample into the flame. flame test formal lab; Your instructor will dip a looped wire into one of the solutions supplied, and then hold it in the bunsen burner flame. the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. Flame tests of metal cations. in conclusion, this lab experiment aimed to determine the characteristic flame colors of various metallic ions in a flame test and understand the. The premise is that heat gives energy to elements and ions, causing them to emit light at a characteristic color or emission spectrum.
From 2020edwardip.weebly.com
Lab Report 1 The Best Flame Edward's Science Awesomeness Flame Lab Conclusion flame test formal lab; Flame tests of metal cations. The premise is that heat gives energy to elements and ions, causing them to emit light at a characteristic color or emission spectrum. Whatever instrument is used to introduce. in conclusion, the flame test lab provided valuable insights into the behavior of metal ions when subjected to. Your instructor. Flame Lab Conclusion.
From athensmutualaid.net
Flame Test Lab Pdf › Athens Mutual Student Corner Flame Lab Conclusion liquids or solids can be tested simply by introducing the sample into the flame. Your instructor will dip a looped wire into one of the solutions supplied, and then hold it in the bunsen burner flame. in conclusion, the flame test lab provided valuable insights into the behavior of metal ions when subjected to. The premise is that. Flame Lab Conclusion.
From studylib.net
Flame Test Exploration Flame Lab Conclusion Sc4 (ldc module #1) chemists began studying colored flames in the 18th century and soon used flame. Your instructor will dip a looped wire into one of the solutions supplied, and then hold it in the bunsen burner flame. the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. . Flame Lab Conclusion.
From www.animalia-life.club
Flame Test Lab Results Flame Lab Conclusion Flame tests of metal cations. Your instructor will dip a looped wire into one of the solutions supplied, and then hold it in the bunsen burner flame. in conclusion, this lab experiment aimed to determine the characteristic flame colors of various metallic ions in a flame test and understand the. The premise is that heat gives energy to elements. Flame Lab Conclusion.
From www.studocu.com
Flame Test Lab Report3 CHEM 1315 Studocu Flame Lab Conclusion Flame tests of metal cations. in conclusion, the flame test lab provided valuable insights into the behavior of metal ions when subjected to. Sc4 (ldc module #1) chemists began studying colored flames in the 18th century and soon used flame. Your instructor will dip a looped wire into one of the solutions supplied, and then hold it in the. Flame Lab Conclusion.
From printablelibbrigg.z21.web.core.windows.net
Flame Test Experiment Lab Report Flame Lab Conclusion liquids or solids can be tested simply by introducing the sample into the flame. flame test formal lab; Your instructor will dip a looped wire into one of the solutions supplied, and then hold it in the bunsen burner flame. Flame tests of metal cations. in conclusion, this lab experiment aimed to determine the characteristic flame colors. Flame Lab Conclusion.
From paperap.com
Flame Test Lab Report Free Essay Example Flame Lab Conclusion Sc4 (ldc module #1) chemists began studying colored flames in the 18th century and soon used flame. the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. in conclusion, this lab experiment aimed to determine the characteristic flame colors of various metallic ions in a flame test and understand. Flame Lab Conclusion.
From ctdmhe.blogspot.com
Megan's Chemistry Lab Blog Lab 7 Flame Test Lab Flame Lab Conclusion Whatever instrument is used to introduce. The premise is that heat gives energy to elements and ions, causing them to emit light at a characteristic color or emission spectrum. flame test formal lab; Flame tests of metal cations. the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. . Flame Lab Conclusion.
From studylib.net
Flame Test Lab. Flame Lab Conclusion Your instructor will dip a looped wire into one of the solutions supplied, and then hold it in the bunsen burner flame. in conclusion, this lab experiment aimed to determine the characteristic flame colors of various metallic ions in a flame test and understand the. Whatever instrument is used to introduce. the flame test is a qualitative test. Flame Lab Conclusion.
From www.studocu.com
Lab report Experiment 1 Flame Test Banawis HJC, Baluyot KJE Flame Lab Conclusion the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. Whatever instrument is used to introduce. in conclusion, this lab experiment aimed to determine the characteristic flame colors of various metallic ions in a flame test and understand the. Sc4 (ldc module #1) chemists began studying colored flames in. Flame Lab Conclusion.
From praxilabs.com
As Featured In Flame Lab Conclusion Your instructor will dip a looped wire into one of the solutions supplied, and then hold it in the bunsen burner flame. the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. in conclusion, this lab experiment aimed to determine the characteristic flame colors of various metallic ions in. Flame Lab Conclusion.
From tukioka-clinic.com
️ Flame test lab report. Flame Test Lab Conclusion by Alexander Stark Flame Lab Conclusion the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. in conclusion, this lab experiment aimed to determine the characteristic flame colors of various metallic ions in a flame test and understand the. Your instructor will dip a looped wire into one of the solutions supplied, and then hold. Flame Lab Conclusion.
From www.youtube.com
Flame Test Lab YouTube Flame Lab Conclusion liquids or solids can be tested simply by introducing the sample into the flame. Your instructor will dip a looped wire into one of the solutions supplied, and then hold it in the bunsen burner flame. The premise is that heat gives energy to elements and ions, causing them to emit light at a characteristic color or emission spectrum.. Flame Lab Conclusion.
From www.slideserve.com
PPT Flame Test Virtual Lab PowerPoint Presentation, free download Flame Lab Conclusion Whatever instrument is used to introduce. liquids or solids can be tested simply by introducing the sample into the flame. the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. The premise is that heat gives energy to elements and ions, causing them to emit light at a characteristic. Flame Lab Conclusion.
From www.animalia-life.club
Flame Test Lab Results Flame Lab Conclusion liquids or solids can be tested simply by introducing the sample into the flame. the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. Sc4 (ldc module #1) chemists began studying colored flames in the 18th century and soon used flame. Your instructor will dip a looped wire into. Flame Lab Conclusion.
From www.scribd.com
Flame Test Lab Example Emission Spectrum Atoms Flame Lab Conclusion the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. The premise is that heat gives energy to elements and ions, causing them to emit light at a characteristic color or emission spectrum. in conclusion, the flame test lab provided valuable insights into the behavior of metal ions when. Flame Lab Conclusion.
From tukioka-clinic.com
️ Flame test lab report. Flame Test Lab Conclusion by Alexander Stark Flame Lab Conclusion in conclusion, this lab experiment aimed to determine the characteristic flame colors of various metallic ions in a flame test and understand the. Sc4 (ldc module #1) chemists began studying colored flames in the 18th century and soon used flame. liquids or solids can be tested simply by introducing the sample into the flame. Whatever instrument is used. Flame Lab Conclusion.
From www.ucc.ie
FLAME Laboratory University College Cork Flame Lab Conclusion Whatever instrument is used to introduce. in conclusion, this lab experiment aimed to determine the characteristic flame colors of various metallic ions in a flame test and understand the. the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. liquids or solids can be tested simply by introducing. Flame Lab Conclusion.
From studylib.net
Flame Test Lab Flame Lab Conclusion Whatever instrument is used to introduce. Your instructor will dip a looped wire into one of the solutions supplied, and then hold it in the bunsen burner flame. liquids or solids can be tested simply by introducing the sample into the flame. in conclusion, the flame test lab provided valuable insights into the behavior of metal ions when. Flame Lab Conclusion.
From studylib.net
Flame Tests Lab Flame Lab Conclusion flame test formal lab; in conclusion, this lab experiment aimed to determine the characteristic flame colors of various metallic ions in a flame test and understand the. in conclusion, the flame test lab provided valuable insights into the behavior of metal ions when subjected to. the flame test is a qualitative test in analytical chemistry used. Flame Lab Conclusion.
From www.youtube.com
How To Flame Test Lab 🔥 YouTube Flame Lab Conclusion Flame tests of metal cations. liquids or solids can be tested simply by introducing the sample into the flame. The premise is that heat gives energy to elements and ions, causing them to emit light at a characteristic color or emission spectrum. Your instructor will dip a looped wire into one of the solutions supplied, and then hold it. Flame Lab Conclusion.
From www.flinnsci.ca
Flame Test Student Laboratory Kit Flame Lab Conclusion in conclusion, this lab experiment aimed to determine the characteristic flame colors of various metallic ions in a flame test and understand the. Sc4 (ldc module #1) chemists began studying colored flames in the 18th century and soon used flame. The premise is that heat gives energy to elements and ions, causing them to emit light at a characteristic. Flame Lab Conclusion.
From www.animalia-life.club
Flame Test Lab Results Flame Lab Conclusion liquids or solids can be tested simply by introducing the sample into the flame. the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. flame test formal lab; in conclusion, this lab experiment aimed to determine the characteristic flame colors of various metallic ions in a flame. Flame Lab Conclusion.
From www.docsity.com
Flame Test Lab Worksheet Activity with Answers Key Exercises Flame Lab Conclusion Your instructor will dip a looped wire into one of the solutions supplied, and then hold it in the bunsen burner flame. the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. in conclusion, the flame test lab provided valuable insights into the behavior of metal ions when subjected. Flame Lab Conclusion.
From hightechhigh-faithsdp.weebly.com
Flame Test Lab Faith's DP Flame Lab Conclusion Whatever instrument is used to introduce. in conclusion, the flame test lab provided valuable insights into the behavior of metal ions when subjected to. Flame tests of metal cations. The premise is that heat gives energy to elements and ions, causing them to emit light at a characteristic color or emission spectrum. Your instructor will dip a looped wire. Flame Lab Conclusion.
From myans.bhantedhammika.net
Flame Test Lab Report Flame Lab Conclusion Whatever instrument is used to introduce. flame test formal lab; liquids or solids can be tested simply by introducing the sample into the flame. Flame tests of metal cations. Your instructor will dip a looped wire into one of the solutions supplied, and then hold it in the bunsen burner flame. The premise is that heat gives energy. Flame Lab Conclusion.
From learningricardo.z19.web.core.windows.net
Flame Test Lab Explanation Flame Lab Conclusion The premise is that heat gives energy to elements and ions, causing them to emit light at a characteristic color or emission spectrum. Sc4 (ldc module #1) chemists began studying colored flames in the 18th century and soon used flame. liquids or solids can be tested simply by introducing the sample into the flame. flame test formal lab;. Flame Lab Conclusion.
From ctdsdcard.blogspot.com
Lab 11 Flame Test Lab Flame Lab Conclusion liquids or solids can be tested simply by introducing the sample into the flame. in conclusion, this lab experiment aimed to determine the characteristic flame colors of various metallic ions in a flame test and understand the. Whatever instrument is used to introduce. flame test formal lab; the flame test is a qualitative test in analytical. Flame Lab Conclusion.
From www.docsity.com
Flame test lab part 2 Study Guides, Projects, Research Chemistry Flame Lab Conclusion flame test formal lab; the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. in conclusion, the flame test lab provided valuable insights into the behavior of metal ions when subjected to. Sc4 (ldc module #1) chemists began studying colored flames in the 18th century and soon used. Flame Lab Conclusion.
From www.studocu.com
LAB Report Flame Test1 OBJECTIVE The experiment conducted aimed to Flame Lab Conclusion The premise is that heat gives energy to elements and ions, causing them to emit light at a characteristic color or emission spectrum. Flame tests of metal cations. in conclusion, the flame test lab provided valuable insights into the behavior of metal ions when subjected to. Your instructor will dip a looped wire into one of the solutions supplied,. Flame Lab Conclusion.
From chemcats.blogspot.com
Chemistry Friday Flame Test Lab Flame Lab Conclusion Whatever instrument is used to introduce. flame test formal lab; Your instructor will dip a looped wire into one of the solutions supplied, and then hold it in the bunsen burner flame. the flame test is a qualitative test in analytical chemistry used to help identify the composition of a sample. liquids or solids can be tested. Flame Lab Conclusion.
From sciencelessonsthatrock.com
Chemistry Flame Test Lab Science Lessons That Rock Flame Lab Conclusion in conclusion, the flame test lab provided valuable insights into the behavior of metal ions when subjected to. in conclusion, this lab experiment aimed to determine the characteristic flame colors of various metallic ions in a flame test and understand the. Sc4 (ldc module #1) chemists began studying colored flames in the 18th century and soon used flame.. Flame Lab Conclusion.
From studylib.net
FLAME TEST LAB PROCEDURE Flame Lab Conclusion Sc4 (ldc module #1) chemists began studying colored flames in the 18th century and soon used flame. in conclusion, this lab experiment aimed to determine the characteristic flame colors of various metallic ions in a flame test and understand the. Flame tests of metal cations. The premise is that heat gives energy to elements and ions, causing them to. Flame Lab Conclusion.
From www.slideshare.net
Flame tests Flame Lab Conclusion in conclusion, this lab experiment aimed to determine the characteristic flame colors of various metallic ions in a flame test and understand the. The premise is that heat gives energy to elements and ions, causing them to emit light at a characteristic color or emission spectrum. flame test formal lab; the flame test is a qualitative test. Flame Lab Conclusion.
From www.academia.edu
(DOC) Lab Report Flame Test Jonathan Ko Academia.edu Flame Lab Conclusion Flame tests of metal cations. Whatever instrument is used to introduce. Your instructor will dip a looped wire into one of the solutions supplied, and then hold it in the bunsen burner flame. Sc4 (ldc module #1) chemists began studying colored flames in the 18th century and soon used flame. liquids or solids can be tested simply by introducing. Flame Lab Conclusion.