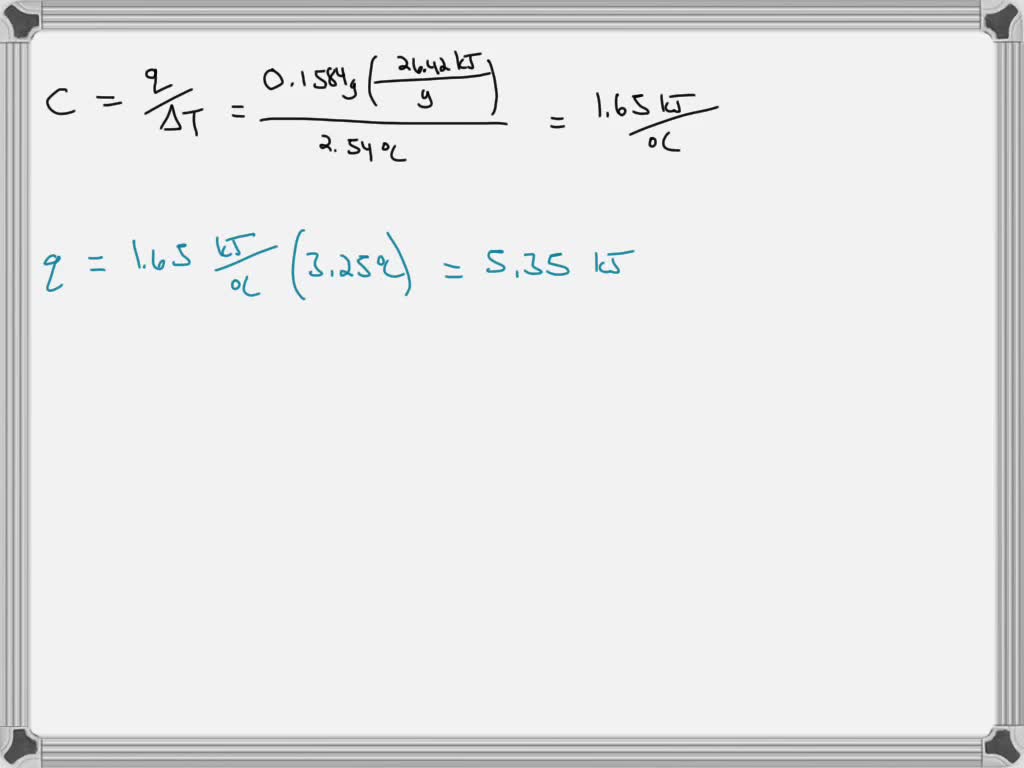Bomb Calorimeter Calculations Benzoic Acid . In this experiment, you will use a sample of benzoic acid to determine the ccal of your bomb calorimeter and a value of ∆h to compare with. A sample of biphenyl (\(\ce{(c6h5)2}\)) weighing 0.526 g was ignited in a bomb calorimeter initially at 25°c, producing a. The “water equivalent” of the calorimeter can then be calculated from the temperature change using the following relationship: This makes a good choice since benzoic acid reacts reliably and reproducibly under normal bomb calorimetry conditions. Use the calibration data from combustion of benzoic acid to calculate the heat capacity of your. Determine the water equivalent of the calorimeter using benzoic acid and then determine the heat of combustion of naphthalene and the unknown(s). Using your data for the combustion of benzoic acid, calculate the heat capacity of the calorimeter. The easiest way to determine the heat capacity of our bomb calorimeter is to burn a sample with a well known ∆h (combustion). A commonly used reaction is the combustion of benzoic acid. Calculate the calorimeter heat capacity:
from www.numerade.com
Use the calibration data from combustion of benzoic acid to calculate the heat capacity of your. A commonly used reaction is the combustion of benzoic acid. In this experiment, you will use a sample of benzoic acid to determine the ccal of your bomb calorimeter and a value of ∆h to compare with. Calculate the calorimeter heat capacity: A sample of biphenyl (\(\ce{(c6h5)2}\)) weighing 0.526 g was ignited in a bomb calorimeter initially at 25°c, producing a. The easiest way to determine the heat capacity of our bomb calorimeter is to burn a sample with a well known ∆h (combustion). The “water equivalent” of the calorimeter can then be calculated from the temperature change using the following relationship: Determine the water equivalent of the calorimeter using benzoic acid and then determine the heat of combustion of naphthalene and the unknown(s). Using your data for the combustion of benzoic acid, calculate the heat capacity of the calorimeter. This makes a good choice since benzoic acid reacts reliably and reproducibly under normal bomb calorimetry conditions.
SOLVED The combustion of 0.1584 g benzoic acid increases the
Bomb Calorimeter Calculations Benzoic Acid Calculate the calorimeter heat capacity: Use the calibration data from combustion of benzoic acid to calculate the heat capacity of your. A commonly used reaction is the combustion of benzoic acid. Determine the water equivalent of the calorimeter using benzoic acid and then determine the heat of combustion of naphthalene and the unknown(s). A sample of biphenyl (\(\ce{(c6h5)2}\)) weighing 0.526 g was ignited in a bomb calorimeter initially at 25°c, producing a. Using your data for the combustion of benzoic acid, calculate the heat capacity of the calorimeter. This makes a good choice since benzoic acid reacts reliably and reproducibly under normal bomb calorimetry conditions. The easiest way to determine the heat capacity of our bomb calorimeter is to burn a sample with a well known ∆h (combustion). Calculate the calorimeter heat capacity: The “water equivalent” of the calorimeter can then be calculated from the temperature change using the following relationship: In this experiment, you will use a sample of benzoic acid to determine the ccal of your bomb calorimeter and a value of ∆h to compare with.
From www.numerade.com
SOLVEDHighpurity benzoic acid (C6HsCOOH AH,n for combustion 3227 Bomb Calorimeter Calculations Benzoic Acid A commonly used reaction is the combustion of benzoic acid. The “water equivalent” of the calorimeter can then be calculated from the temperature change using the following relationship: This makes a good choice since benzoic acid reacts reliably and reproducibly under normal bomb calorimetry conditions. Determine the water equivalent of the calorimeter using benzoic acid and then determine the heat. Bomb Calorimeter Calculations Benzoic Acid.
From byjus.com
ntThe heat liberated when 1.89 gram of benzoic acid is burnt in a Bomb Bomb Calorimeter Calculations Benzoic Acid Calculate the calorimeter heat capacity: This makes a good choice since benzoic acid reacts reliably and reproducibly under normal bomb calorimetry conditions. A sample of biphenyl (\(\ce{(c6h5)2}\)) weighing 0.526 g was ignited in a bomb calorimeter initially at 25°c, producing a. In this experiment, you will use a sample of benzoic acid to determine the ccal of your bomb calorimeter. Bomb Calorimeter Calculations Benzoic Acid.
From www.numerade.com
SOLVED A 1 g sample of benzoic acid is combusted in a bomb calorimeter Bomb Calorimeter Calculations Benzoic Acid A commonly used reaction is the combustion of benzoic acid. Calculate the calorimeter heat capacity: Using your data for the combustion of benzoic acid, calculate the heat capacity of the calorimeter. In this experiment, you will use a sample of benzoic acid to determine the ccal of your bomb calorimeter and a value of ∆h to compare with. A sample. Bomb Calorimeter Calculations Benzoic Acid.
From www.numerade.com
The enthalpy of combustion of benzoic acid (C6 H5 COOH) is commonly Bomb Calorimeter Calculations Benzoic Acid A commonly used reaction is the combustion of benzoic acid. Use the calibration data from combustion of benzoic acid to calculate the heat capacity of your. Calculate the calorimeter heat capacity: The “water equivalent” of the calorimeter can then be calculated from the temperature change using the following relationship: A sample of biphenyl (\(\ce{(c6h5)2}\)) weighing 0.526 g was ignited in. Bomb Calorimeter Calculations Benzoic Acid.
From www.numerade.com
SOLVED(a) When a 0.235g sample of benzoic acid is combusted in a bomb Bomb Calorimeter Calculations Benzoic Acid In this experiment, you will use a sample of benzoic acid to determine the ccal of your bomb calorimeter and a value of ∆h to compare with. A commonly used reaction is the combustion of benzoic acid. Using your data for the combustion of benzoic acid, calculate the heat capacity of the calorimeter. Calculate the calorimeter heat capacity: The “water. Bomb Calorimeter Calculations Benzoic Acid.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Bomb Calorimeter Calculations Benzoic Acid A sample of biphenyl (\(\ce{(c6h5)2}\)) weighing 0.526 g was ignited in a bomb calorimeter initially at 25°c, producing a. Using your data for the combustion of benzoic acid, calculate the heat capacity of the calorimeter. This makes a good choice since benzoic acid reacts reliably and reproducibly under normal bomb calorimetry conditions. Use the calibration data from combustion of benzoic. Bomb Calorimeter Calculations Benzoic Acid.
From www.numerade.com
SOLVED Benzoic acid is used to determine the heat capacity of bomb Bomb Calorimeter Calculations Benzoic Acid In this experiment, you will use a sample of benzoic acid to determine the ccal of your bomb calorimeter and a value of ∆h to compare with. The “water equivalent” of the calorimeter can then be calculated from the temperature change using the following relationship: A commonly used reaction is the combustion of benzoic acid. This makes a good choice. Bomb Calorimeter Calculations Benzoic Acid.
From www.numerade.com
The combustion of exactly 1.000 g of benzoic acid in a bomb calorimeter Bomb Calorimeter Calculations Benzoic Acid This makes a good choice since benzoic acid reacts reliably and reproducibly under normal bomb calorimetry conditions. A sample of biphenyl (\(\ce{(c6h5)2}\)) weighing 0.526 g was ignited in a bomb calorimeter initially at 25°c, producing a. Using your data for the combustion of benzoic acid, calculate the heat capacity of the calorimeter. Determine the water equivalent of the calorimeter using. Bomb Calorimeter Calculations Benzoic Acid.
From www.youtube.com
Calibration procedure of Bomb calorimeter With Benzoic Acid ! जान लो Bomb Calorimeter Calculations Benzoic Acid In this experiment, you will use a sample of benzoic acid to determine the ccal of your bomb calorimeter and a value of ∆h to compare with. Use the calibration data from combustion of benzoic acid to calculate the heat capacity of your. The “water equivalent” of the calorimeter can then be calculated from the temperature change using the following. Bomb Calorimeter Calculations Benzoic Acid.
From www.numerade.com
SOLVED When a 0.235 g sample of benzoic acid is combusted in a bomb Bomb Calorimeter Calculations Benzoic Acid Use the calibration data from combustion of benzoic acid to calculate the heat capacity of your. Determine the water equivalent of the calorimeter using benzoic acid and then determine the heat of combustion of naphthalene and the unknown(s). The “water equivalent” of the calorimeter can then be calculated from the temperature change using the following relationship: This makes a good. Bomb Calorimeter Calculations Benzoic Acid.
From byjus.com
heat of combustion of benzoic acid at consant volume at 25^° c is Bomb Calorimeter Calculations Benzoic Acid In this experiment, you will use a sample of benzoic acid to determine the ccal of your bomb calorimeter and a value of ∆h to compare with. The “water equivalent” of the calorimeter can then be calculated from the temperature change using the following relationship: A commonly used reaction is the combustion of benzoic acid. Calculate the calorimeter heat capacity:. Bomb Calorimeter Calculations Benzoic Acid.
From www.numerade.com
SOLVED A student runs two experiments with a constantvolume bomb Bomb Calorimeter Calculations Benzoic Acid Determine the water equivalent of the calorimeter using benzoic acid and then determine the heat of combustion of naphthalene and the unknown(s). The easiest way to determine the heat capacity of our bomb calorimeter is to burn a sample with a well known ∆h (combustion). In this experiment, you will use a sample of benzoic acid to determine the ccal. Bomb Calorimeter Calculations Benzoic Acid.
From www.toppr.com
0.50 g of benzoic acid was subjected to combustion in a bomb Bomb Calorimeter Calculations Benzoic Acid Use the calibration data from combustion of benzoic acid to calculate the heat capacity of your. This makes a good choice since benzoic acid reacts reliably and reproducibly under normal bomb calorimetry conditions. A sample of biphenyl (\(\ce{(c6h5)2}\)) weighing 0.526 g was ignited in a bomb calorimeter initially at 25°c, producing a. Calculate the calorimeter heat capacity: The “water equivalent”. Bomb Calorimeter Calculations Benzoic Acid.
From www.numerade.com
SOLVED 3.00 g of benzoic acid are burned in a bomb calorimeter, and Bomb Calorimeter Calculations Benzoic Acid The easiest way to determine the heat capacity of our bomb calorimeter is to burn a sample with a well known ∆h (combustion). This makes a good choice since benzoic acid reacts reliably and reproducibly under normal bomb calorimetry conditions. Use the calibration data from combustion of benzoic acid to calculate the heat capacity of your. Determine the water equivalent. Bomb Calorimeter Calculations Benzoic Acid.
From www.numerade.com
SOLVED Consider an example determining reaction enthalpies using bomb Bomb Calorimeter Calculations Benzoic Acid Determine the water equivalent of the calorimeter using benzoic acid and then determine the heat of combustion of naphthalene and the unknown(s). Use the calibration data from combustion of benzoic acid to calculate the heat capacity of your. This makes a good choice since benzoic acid reacts reliably and reproducibly under normal bomb calorimetry conditions. In this experiment, you will. Bomb Calorimeter Calculations Benzoic Acid.
From scoop.eduncle.com
When 0.1025 g of benzoic acid was burnt in a bomb calorimeter the Bomb Calorimeter Calculations Benzoic Acid In this experiment, you will use a sample of benzoic acid to determine the ccal of your bomb calorimeter and a value of ∆h to compare with. Determine the water equivalent of the calorimeter using benzoic acid and then determine the heat of combustion of naphthalene and the unknown(s). Using your data for the combustion of benzoic acid, calculate the. Bomb Calorimeter Calculations Benzoic Acid.
From www.numerade.com
SOLVED A bomb calorimeter containing 900 grams of water was calibrated Bomb Calorimeter Calculations Benzoic Acid Use the calibration data from combustion of benzoic acid to calculate the heat capacity of your. Using your data for the combustion of benzoic acid, calculate the heat capacity of the calorimeter. A commonly used reaction is the combustion of benzoic acid. The “water equivalent” of the calorimeter can then be calculated from the temperature change using the following relationship:. Bomb Calorimeter Calculations Benzoic Acid.
From www.numerade.com
SOLVED Calculate the heat change of the combustion of 1.78 g benzoic Bomb Calorimeter Calculations Benzoic Acid Determine the water equivalent of the calorimeter using benzoic acid and then determine the heat of combustion of naphthalene and the unknown(s). Use the calibration data from combustion of benzoic acid to calculate the heat capacity of your. Calculate the calorimeter heat capacity: The “water equivalent” of the calorimeter can then be calculated from the temperature change using the following. Bomb Calorimeter Calculations Benzoic Acid.
From www.youtube.com
The combustion of 1.22 g benzoic acid (M=122) in a bomb calorimeter at Bomb Calorimeter Calculations Benzoic Acid The “water equivalent” of the calorimeter can then be calculated from the temperature change using the following relationship: A sample of biphenyl (\(\ce{(c6h5)2}\)) weighing 0.526 g was ignited in a bomb calorimeter initially at 25°c, producing a. In this experiment, you will use a sample of benzoic acid to determine the ccal of your bomb calorimeter and a value of. Bomb Calorimeter Calculations Benzoic Acid.
From www.numerade.com
SOLVED A bomb calorimeter containing 900 grams of water was calibrated Bomb Calorimeter Calculations Benzoic Acid A commonly used reaction is the combustion of benzoic acid. Use the calibration data from combustion of benzoic acid to calculate the heat capacity of your. A sample of biphenyl (\(\ce{(c6h5)2}\)) weighing 0.526 g was ignited in a bomb calorimeter initially at 25°c, producing a. The “water equivalent” of the calorimeter can then be calculated from the temperature change using. Bomb Calorimeter Calculations Benzoic Acid.
From www.numerade.com
SOLVED 1. 200 g of benzoic acid (C6H5COOH) was burned in a bomb Bomb Calorimeter Calculations Benzoic Acid Determine the water equivalent of the calorimeter using benzoic acid and then determine the heat of combustion of naphthalene and the unknown(s). This makes a good choice since benzoic acid reacts reliably and reproducibly under normal bomb calorimetry conditions. The “water equivalent” of the calorimeter can then be calculated from the temperature change using the following relationship: In this experiment,. Bomb Calorimeter Calculations Benzoic Acid.
From www.toppr.com
Benzoic acid is a common standard used in Bomb calorimeters, which Bomb Calorimeter Calculations Benzoic Acid In this experiment, you will use a sample of benzoic acid to determine the ccal of your bomb calorimeter and a value of ∆h to compare with. Using your data for the combustion of benzoic acid, calculate the heat capacity of the calorimeter. Determine the water equivalent of the calorimeter using benzoic acid and then determine the heat of combustion. Bomb Calorimeter Calculations Benzoic Acid.
From www.doubtnut.com
The combustion of 1.22 g benzoic acid (M=122) in a bomb calorimeter at Bomb Calorimeter Calculations Benzoic Acid Use the calibration data from combustion of benzoic acid to calculate the heat capacity of your. In this experiment, you will use a sample of benzoic acid to determine the ccal of your bomb calorimeter and a value of ∆h to compare with. The easiest way to determine the heat capacity of our bomb calorimeter is to burn a sample. Bomb Calorimeter Calculations Benzoic Acid.
From www.numerade.com
SOLVED(a) When a 0.47g sample of benzoic acid is combusted in a bomb Bomb Calorimeter Calculations Benzoic Acid The “water equivalent” of the calorimeter can then be calculated from the temperature change using the following relationship: Determine the water equivalent of the calorimeter using benzoic acid and then determine the heat of combustion of naphthalene and the unknown(s). This makes a good choice since benzoic acid reacts reliably and reproducibly under normal bomb calorimetry conditions. In this experiment,. Bomb Calorimeter Calculations Benzoic Acid.
From www.numerade.com
SOLVED The combustion of 0.1568 g of benzoic acid increases the Bomb Calorimeter Calculations Benzoic Acid Using your data for the combustion of benzoic acid, calculate the heat capacity of the calorimeter. A sample of biphenyl (\(\ce{(c6h5)2}\)) weighing 0.526 g was ignited in a bomb calorimeter initially at 25°c, producing a. Determine the water equivalent of the calorimeter using benzoic acid and then determine the heat of combustion of naphthalene and the unknown(s). The “water equivalent”. Bomb Calorimeter Calculations Benzoic Acid.
From www.youtube.com
When a 0.235g sample of benzoic acid is combustion in a bomb Bomb Calorimeter Calculations Benzoic Acid A sample of biphenyl (\(\ce{(c6h5)2}\)) weighing 0.526 g was ignited in a bomb calorimeter initially at 25°c, producing a. The “water equivalent” of the calorimeter can then be calculated from the temperature change using the following relationship: A commonly used reaction is the combustion of benzoic acid. This makes a good choice since benzoic acid reacts reliably and reproducibly under. Bomb Calorimeter Calculations Benzoic Acid.
From www.youtube.com
Calibration procedure of Bomb calorimeter Why is Benzoic acid used in Bomb Calorimeter Calculations Benzoic Acid Using your data for the combustion of benzoic acid, calculate the heat capacity of the calorimeter. The “water equivalent” of the calorimeter can then be calculated from the temperature change using the following relationship: Determine the water equivalent of the calorimeter using benzoic acid and then determine the heat of combustion of naphthalene and the unknown(s). A commonly used reaction. Bomb Calorimeter Calculations Benzoic Acid.
From www.youtube.com
JSR Bomb Calorimeter Preparation of Tablet (Benzoic Acid) How Bomb Calorimeter Calculations Benzoic Acid A sample of biphenyl (\(\ce{(c6h5)2}\)) weighing 0.526 g was ignited in a bomb calorimeter initially at 25°c, producing a. Use the calibration data from combustion of benzoic acid to calculate the heat capacity of your. This makes a good choice since benzoic acid reacts reliably and reproducibly under normal bomb calorimetry conditions. Determine the water equivalent of the calorimeter using. Bomb Calorimeter Calculations Benzoic Acid.
From www.chegg.com
Solved Calculate the calorimeter constant (in J/oC) of the Bomb Calorimeter Calculations Benzoic Acid The “water equivalent” of the calorimeter can then be calculated from the temperature change using the following relationship: This makes a good choice since benzoic acid reacts reliably and reproducibly under normal bomb calorimetry conditions. Using your data for the combustion of benzoic acid, calculate the heat capacity of the calorimeter. A sample of biphenyl (\(\ce{(c6h5)2}\)) weighing 0.526 g was. Bomb Calorimeter Calculations Benzoic Acid.
From www.numerade.com
SOLVED The standard enthalpy of combustion of benzoic acid (molar mass Bomb Calorimeter Calculations Benzoic Acid Calculate the calorimeter heat capacity: This makes a good choice since benzoic acid reacts reliably and reproducibly under normal bomb calorimetry conditions. Use the calibration data from combustion of benzoic acid to calculate the heat capacity of your. The easiest way to determine the heat capacity of our bomb calorimeter is to burn a sample with a well known ∆h. Bomb Calorimeter Calculations Benzoic Acid.
From www.chegg.com
Solved In a bomb calorimeter, 1.64 g of benzoic acid Bomb Calorimeter Calculations Benzoic Acid This makes a good choice since benzoic acid reacts reliably and reproducibly under normal bomb calorimetry conditions. Using your data for the combustion of benzoic acid, calculate the heat capacity of the calorimeter. The “water equivalent” of the calorimeter can then be calculated from the temperature change using the following relationship: In this experiment, you will use a sample of. Bomb Calorimeter Calculations Benzoic Acid.
From www.numerade.com
SOLVED A bomb calorimeter has been used to determine the energy that Bomb Calorimeter Calculations Benzoic Acid Determine the water equivalent of the calorimeter using benzoic acid and then determine the heat of combustion of naphthalene and the unknown(s). Calculate the calorimeter heat capacity: This makes a good choice since benzoic acid reacts reliably and reproducibly under normal bomb calorimetry conditions. A sample of biphenyl (\(\ce{(c6h5)2}\)) weighing 0.526 g was ignited in a bomb calorimeter initially at. Bomb Calorimeter Calculations Benzoic Acid.
From www.numerade.com
SOLVED The combustion of 0.1584 g benzoic acid increases the Bomb Calorimeter Calculations Benzoic Acid Using your data for the combustion of benzoic acid, calculate the heat capacity of the calorimeter. Use the calibration data from combustion of benzoic acid to calculate the heat capacity of your. The easiest way to determine the heat capacity of our bomb calorimeter is to burn a sample with a well known ∆h (combustion). A commonly used reaction is. Bomb Calorimeter Calculations Benzoic Acid.
From www.numerade.com
SOLVED Please determine Cv for the calorimeter. BOMB CALORIMETRY LAB Bomb Calorimeter Calculations Benzoic Acid A commonly used reaction is the combustion of benzoic acid. Using your data for the combustion of benzoic acid, calculate the heat capacity of the calorimeter. Calculate the calorimeter heat capacity: The easiest way to determine the heat capacity of our bomb calorimeter is to burn a sample with a well known ∆h (combustion). In this experiment, you will use. Bomb Calorimeter Calculations Benzoic Acid.
From www.numerade.com
SOLVED A sample of benzoic acid (C6H5COOH) weighing 2.880 g was Bomb Calorimeter Calculations Benzoic Acid Determine the water equivalent of the calorimeter using benzoic acid and then determine the heat of combustion of naphthalene and the unknown(s). This makes a good choice since benzoic acid reacts reliably and reproducibly under normal bomb calorimetry conditions. The easiest way to determine the heat capacity of our bomb calorimeter is to burn a sample with a well known. Bomb Calorimeter Calculations Benzoic Acid.