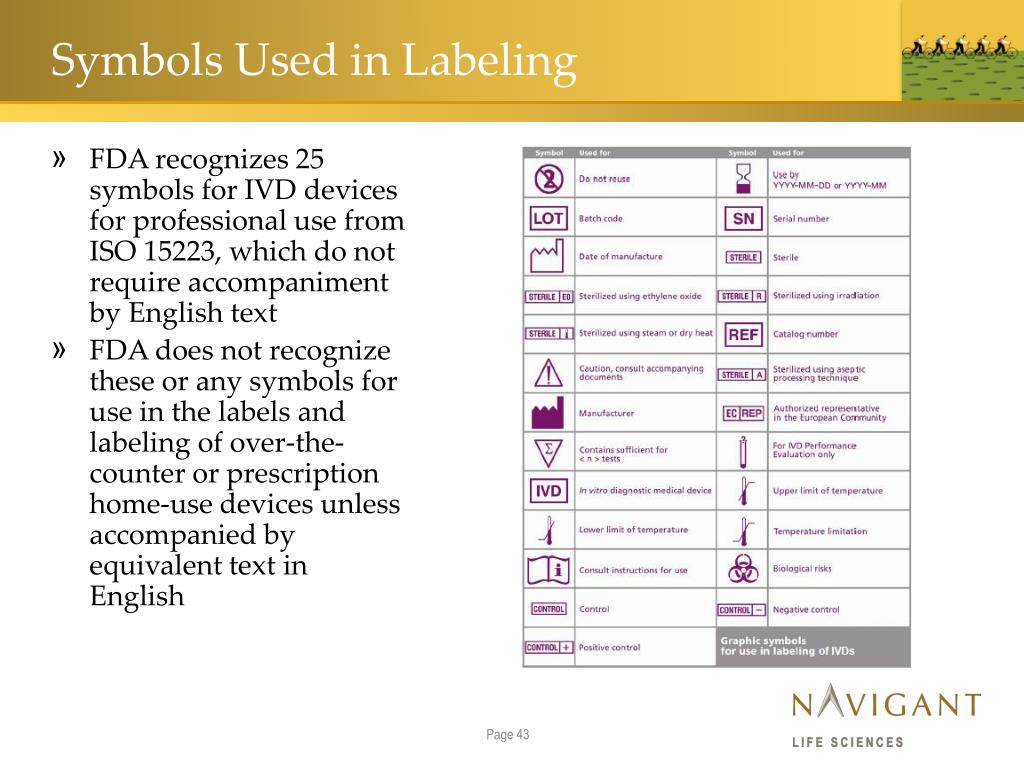
Fda Off Label Use Device . Use of a marketed product in this manner when the intent is the practice of medicine does not require the submission of an investigational. It would contradict this statutory scheme for fda to clear or approve a device with a particular design or composition and then turn. On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. Must an irb review a study.
from mungfali.com
It would contradict this statutory scheme for fda to clear or approve a device with a particular design or composition and then turn. Use of a marketed product in this manner when the intent is the practice of medicine does not require the submission of an investigational. On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. Must an irb review a study.
Medical Device Labeling Symbols
Fda Off Label Use Device Must an irb review a study. It would contradict this statutory scheme for fda to clear or approve a device with a particular design or composition and then turn. On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. Use of a marketed product in this manner when the intent is the practice of medicine does not require the submission of an investigational. Must an irb review a study.
From drugpricing.norc.org
OffLabel Drugs Same Treatment, Different Costs Drug Pricing Explained Fda Off Label Use Device It would contradict this statutory scheme for fda to clear or approve a device with a particular design or composition and then turn. Use of a marketed product in this manner when the intent is the practice of medicine does not require the submission of an investigational. Must an irb review a study. On tuesday, october 24, fda announced the. Fda Off Label Use Device.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download Fda Off Label Use Device Use of a marketed product in this manner when the intent is the practice of medicine does not require the submission of an investigational. It would contradict this statutory scheme for fda to clear or approve a device with a particular design or composition and then turn. Must an irb review a study. On tuesday, october 24, fda announced the. Fda Off Label Use Device.
From www.tailoredlabel.com
UDI Label Requirements For FDA Medical Device Labels TLP Fda Off Label Use Device It would contradict this statutory scheme for fda to clear or approve a device with a particular design or composition and then turn. On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. Must an irb review a study. Use of a marketed product in this manner when the intent is the practice of. Fda Off Label Use Device.
From www.pharmaceutical-technology.com
FDA cracks down on offlabel drug use messaging Pharmaceutical Technology Fda Off Label Use Device It would contradict this statutory scheme for fda to clear or approve a device with a particular design or composition and then turn. Must an irb review a study. Use of a marketed product in this manner when the intent is the practice of medicine does not require the submission of an investigational. On tuesday, october 24, fda announced the. Fda Off Label Use Device.
From www.regdesk.co
FDA on General Principles of Labeling for Medical Devices RegDesk Fda Off Label Use Device Use of a marketed product in this manner when the intent is the practice of medicine does not require the submission of an investigational. It would contradict this statutory scheme for fda to clear or approve a device with a particular design or composition and then turn. On tuesday, october 24, fda announced the availability of a revised draft guidance. Fda Off Label Use Device.
From www.medicaldesignandoutsourcing.com
FDA to clarify role of offlabel uses in medical device approvals Fda Off Label Use Device Must an irb review a study. It would contradict this statutory scheme for fda to clear or approve a device with a particular design or composition and then turn. On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. Use of a marketed product in this manner when the intent is the practice of. Fda Off Label Use Device.
From mungfali.com
FDA Medical Device Label Symbols Fda Off Label Use Device Use of a marketed product in this manner when the intent is the practice of medicine does not require the submission of an investigational. On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. It would contradict this statutory scheme for fda to clear or approve a device with a particular design or composition. Fda Off Label Use Device.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Fda Off Label Use Device On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. It would contradict this statutory scheme for fda to clear or approve a device with a particular design or composition and then turn. Use of a marketed product in this manner when the intent is the practice of medicine does not require the submission. Fda Off Label Use Device.
From www.pm360online.com
What Does the FDA’s New Offlabel Guidance Mean for Pharma? PM360 Fda Off Label Use Device It would contradict this statutory scheme for fda to clear or approve a device with a particular design or composition and then turn. On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. Use of a marketed product in this manner when the intent is the practice of medicine does not require the submission. Fda Off Label Use Device.
From emmainternational.com
Discovering FDALabel Your GoTo Labelling Tool Fda Off Label Use Device On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. It would contradict this statutory scheme for fda to clear or approve a device with a particular design or composition and then turn. Use of a marketed product in this manner when the intent is the practice of medicine does not require the submission. Fda Off Label Use Device.
From www.alto.com
OffLabel Medication Use, Explained Fda Off Label Use Device It would contradict this statutory scheme for fda to clear or approve a device with a particular design or composition and then turn. Use of a marketed product in this manner when the intent is the practice of medicine does not require the submission of an investigational. Must an irb review a study. On tuesday, october 24, fda announced the. Fda Off Label Use Device.
From www.youtube.com
FDA's Role in the Off Label Use of Medical Devices Explained by Dr Fda Off Label Use Device Must an irb review a study. On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. It would contradict this statutory scheme for fda to clear or approve a device with a particular design or composition and then turn. Use of a marketed product in this manner when the intent is the practice of. Fda Off Label Use Device.
From mungfali.com
Medical Device Labeling Symbols Fda Off Label Use Device Must an irb review a study. On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. It would contradict this statutory scheme for fda to clear or approve a device with a particular design or composition and then turn. Use of a marketed product in this manner when the intent is the practice of. Fda Off Label Use Device.
From www.jacc.org
OffLabel Use vs OffLabel Marketing of Drugs Part 1 OffLabel Use Fda Off Label Use Device Must an irb review a study. It would contradict this statutory scheme for fda to clear or approve a device with a particular design or composition and then turn. On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. Use of a marketed product in this manner when the intent is the practice of. Fda Off Label Use Device.
From www.druganddevicelawblog.com
Examining The Amarin/FDA OffLabel Promotion Settlement Drug & Device Law Fda Off Label Use Device On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. Must an irb review a study. It would contradict this statutory scheme for fda to clear or approve a device with a particular design or composition and then turn. Use of a marketed product in this manner when the intent is the practice of. Fda Off Label Use Device.
From www.audible.com
FDA's Role in the Off Label Use of Medical Devices Explained by Dr Fda Off Label Use Device Use of a marketed product in this manner when the intent is the practice of medicine does not require the submission of an investigational. It would contradict this statutory scheme for fda to clear or approve a device with a particular design or composition and then turn. Must an irb review a study. On tuesday, october 24, fda announced the. Fda Off Label Use Device.
From www.vrogue.co
Fda Medical Device Labeling Requirements Presentation vrogue.co Fda Off Label Use Device On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. Must an irb review a study. It would contradict this statutory scheme for fda to clear or approve a device with a particular design or composition and then turn. Use of a marketed product in this manner when the intent is the practice of. Fda Off Label Use Device.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Fda Off Label Use Device Must an irb review a study. Use of a marketed product in this manner when the intent is the practice of medicine does not require the submission of an investigational. On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. It would contradict this statutory scheme for fda to clear or approve a device. Fda Off Label Use Device.
From www.regdesk.co
FDA Guidance on Development of Medical Device Labeling RegDesk Fda Off Label Use Device Use of a marketed product in this manner when the intent is the practice of medicine does not require the submission of an investigational. Must an irb review a study. It would contradict this statutory scheme for fda to clear or approve a device with a particular design or composition and then turn. On tuesday, october 24, fda announced the. Fda Off Label Use Device.
From studylib.net
OFFLABEL USE OF MEDICAL PRODUCTS WARRANTY AND Fda Off Label Use Device Must an irb review a study. Use of a marketed product in this manner when the intent is the practice of medicine does not require the submission of an investigational. On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. It would contradict this statutory scheme for fda to clear or approve a device. Fda Off Label Use Device.
From www.vrogue.co
Fda Medical Device Label Symbols vrogue.co Fda Off Label Use Device Use of a marketed product in this manner when the intent is the practice of medicine does not require the submission of an investigational. On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. Must an irb review a study. It would contradict this statutory scheme for fda to clear or approve a device. Fda Off Label Use Device.
From www.youtube.com
Good OffLabel Use Practice of Medicines YouTube Fda Off Label Use Device Use of a marketed product in this manner when the intent is the practice of medicine does not require the submission of an investigational. On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. Must an irb review a study. It would contradict this statutory scheme for fda to clear or approve a device. Fda Off Label Use Device.
From dandelionsandthings.blogspot.com
34 Epidiolex Off Label Use Label Design Ideas 2020 Fda Off Label Use Device On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. Use of a marketed product in this manner when the intent is the practice of medicine does not require the submission of an investigational. It would contradict this statutory scheme for fda to clear or approve a device with a particular design or composition. Fda Off Label Use Device.
From mungfali.com
FDA Medical Device Label Symbols Fda Off Label Use Device On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. It would contradict this statutory scheme for fda to clear or approve a device with a particular design or composition and then turn. Use of a marketed product in this manner when the intent is the practice of medicine does not require the submission. Fda Off Label Use Device.
From www.tapecon.com
What Information Should You Include on Your Medical Device Label? Fda Off Label Use Device On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. It would contradict this statutory scheme for fda to clear or approve a device with a particular design or composition and then turn. Use of a marketed product in this manner when the intent is the practice of medicine does not require the submission. Fda Off Label Use Device.
From doctorstipsonline.com
On Label and Off Label Uses Of Best Microcurrent Device Fda Off Label Use Device Must an irb review a study. On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. It would contradict this statutory scheme for fda to clear or approve a device with a particular design or composition and then turn. Use of a marketed product in this manner when the intent is the practice of. Fda Off Label Use Device.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Fda Off Label Use Device On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. It would contradict this statutory scheme for fda to clear or approve a device with a particular design or composition and then turn. Use of a marketed product in this manner when the intent is the practice of medicine does not require the submission. Fda Off Label Use Device.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Fda Off Label Use Device Must an irb review a study. On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. Use of a marketed product in this manner when the intent is the practice of medicine does not require the submission of an investigational. It would contradict this statutory scheme for fda to clear or approve a device. Fda Off Label Use Device.
From stellacenter.com
OffLabel Medical Treatments Proven Effective Stella Fda Off Label Use Device Use of a marketed product in this manner when the intent is the practice of medicine does not require the submission of an investigational. On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. It would contradict this statutory scheme for fda to clear or approve a device with a particular design or composition. Fda Off Label Use Device.
From www.vrogue.co
Medical Device Labeling Requirements What You Need To vrogue.co Fda Off Label Use Device Must an irb review a study. It would contradict this statutory scheme for fda to clear or approve a device with a particular design or composition and then turn. Use of a marketed product in this manner when the intent is the practice of medicine does not require the submission of an investigational. On tuesday, october 24, fda announced the. Fda Off Label Use Device.
From www.slideshare.net
FDA Unique Device Identification (UDI) Overview Fda Off Label Use Device Must an irb review a study. It would contradict this statutory scheme for fda to clear or approve a device with a particular design or composition and then turn. On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. Use of a marketed product in this manner when the intent is the practice of. Fda Off Label Use Device.
From www.meddeviceonline.com
Medical Device Labeling New ISO 152231 FDA Guidance UDI Fda Off Label Use Device Use of a marketed product in this manner when the intent is the practice of medicine does not require the submission of an investigational. Must an irb review a study. It would contradict this statutory scheme for fda to clear or approve a device with a particular design or composition and then turn. On tuesday, october 24, fda announced the. Fda Off Label Use Device.
From exoyainpv.blob.core.windows.net
Medical Device OffLabel Usage at Robert Salazar blog Fda Off Label Use Device Must an irb review a study. On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. Use of a marketed product in this manner when the intent is the practice of medicine does not require the submission of an investigational. It would contradict this statutory scheme for fda to clear or approve a device. Fda Off Label Use Device.
From www.researchgate.net
IL6 agents FDA approved and offlabel uses. Download Scientific Diagram Fda Off Label Use Device Use of a marketed product in this manner when the intent is the practice of medicine does not require the submission of an investigational. It would contradict this statutory scheme for fda to clear or approve a device with a particular design or composition and then turn. On tuesday, october 24, fda announced the availability of a revised draft guidance. Fda Off Label Use Device.
From www.northcarolinaproductliabilitylawyer.com
OffLabel Use Category Archives — North Carolina Product Liability Fda Off Label Use Device On tuesday, october 24, fda announced the availability of a revised draft guidance document entitled “communications. It would contradict this statutory scheme for fda to clear or approve a device with a particular design or composition and then turn. Must an irb review a study. Use of a marketed product in this manner when the intent is the practice of. Fda Off Label Use Device.